Accelerating Therapeutic Development for Brain Illnesses
IRP Study Finds Brain Imaging Technique Could Aid Clinical Trials
IRP researchers used magnetic resonance imaging (MRI) to track changes in brain connections in children with a rare neurological disease, suggesting the approach could help them more quickly figure out whether treatments for the condition work.
We all crave instant gratification, but unfortunately, most things worth doing take time. This certainly applies to evaluating treatments for many illnesses, as observable changes in patients’ symptoms can take weeks or months to appear. Fortunately, new NIH research has revealed that a particular way of non-invasively peering into the brain could help accelerate the development of treatments for a variety of rare neurological diseases.1
Among the many wonders of the human body, one of the most important is the way the tiny neurons in our brains work together to allow us to move and speak. Sadly, in children with a rare disease called GM1 gangliosidosis, a toxic buildup of a protein called GM1 ganglioside kills those hard-working cells, causing the gradual degeneration of those abilities. The disease results from mutations that disable both copies of the GLB1 gene, without which cells are unable to break down that protein.
“GM1 ganglioside is more prevalent in neurons than in other cells, which is why the disease is so destructive to the central nervous system,” explains the paper’s first author, Connor J. Lewis, who recently finished a two-year stint as a postbaccalaureate fellow in the lab of IRP associate investigator Maria T. Acosta, M.D.
There is no treatment for the disease, so people with the juvenile-onset form that Dr. Acosta and her IRP colleagues focus on generally die in their teens or early 20s. There may be a light on the horizon for such patients, though: a gene therapy that gives patients a normal copy of the GLB1 gene, which was developed by researchers at Auburn University and the University of Massachusetts’ Chan Medical School, where Connor is now pursuing both an M.D. and a Ph.D. However, as difficult as creating the therapy was, proving that it works in actual patients is another challenge entirely.
To that end, an IRP research team led by IRP senior clinician Cynthia Tifft, M.D., Ph.D., began running a clinical trial testing the therapy at the NIH Clinical Center in 2019. To help that process move as swiftly and efficiently as possible, the group is relying not just on doctors’ evaluations of patients’ symptoms, but also magnetic resonance imaging (MRI) techniques that allow them to track changes in patients’ brains over time.

Dr. Maria T. Acosta, the new MRI study’s senior author (left), and Connor J. Lewis, the study’s lead author (right)
“Any clinical trial to test a potential treatment requires some evaluation that indicates whether there is a change due to the treatment,” says Dr. Acosta, who is leading the MRI component of the IRP effort. “For some conditions, and for some treatments, clinical improvements can take a lot of time, and sometimes clinicians and pharmaceutical companies don’t have the time to be able to wait for clinical improvement. You can imagine that if a patient took ten years for their brain connections to degenerate, it could take ten years for their symptoms to improve. Neither the clinicians nor the companies will have the time to wait for those improvements, so we need techniques that can be used as biomarkers to show this improvement. MRI techniques, in general, are a very good window to assess brain changes, and they are not invasive.”
The new study that Connor and Dr. Acosta spearheaded specifically utilized an MRI analysis technique called differential tractography, which provides a measure of how many connections between different parts of the brain appear or disappear over time, as well as how existing connections grow and shrink. When the researchers compared those changes in patients with GM1 gangliosidosis and children of the same age without the disease, they found that the number and size of neuronal superhighways decreased over time in the patients and increased over time in the healthy children.
“In any given period of time, children gain and lose neurons,” Dr. Acosta says. “That’s normal in development. We needed to be sure that the observed changes — gains or losses — were different than those you would expect in the general population.”
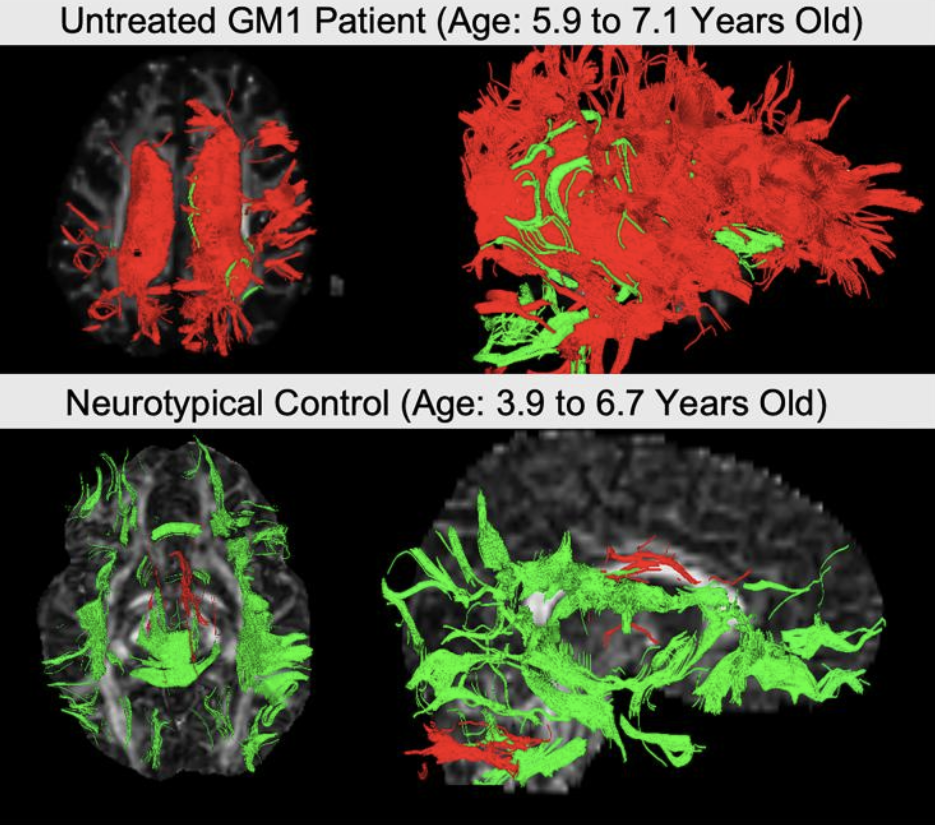
These two images from the study, produced using differential tractography, show neuronal connections that shrank (red) or grew (green) over time in a child with GM1 gangliosidosis (top) and a healthy child (bottom).
What’s more, young patients whose movement, speaking, and cognitive capabilities deteriorated more over time tended to have larger decreases in the number and size of the brain connections differential tractography tracks. The finding strongly suggests those changes in the brain are directly connected to patient’ symptoms, so if a treatment slows, stops, or reverses the changes, researchers can be optimistic that it is working even if improvements in symptoms have not yet become significant enough for physicians to observe. That could help shorten the time needed to complete a clinical trial, thereby accelerating the development of treatments not just for GM1 gangliosidosis, but also numerous other diseases that destroy neurons.
“In a very short amount of time, using some MRI scans, we should be able to determine if we are seeing changes or not, and that will give us an idea of how we should move forward in a clinical trial in the future,” Dr. Acosta explains.

Swedish siblings Isabella, Julia, and Hampus Flysjo (left to right), seen here with their parents Jessica and Niclas, all received the GM1 gangliosidosis gene therapy as part of the NIH clinical trial. Both clinical evaluations and brain imaging techniques like differential tractography suggest that the treatment has helped protect them from the disease’s effects. Photo courtesy of Niclas Flysjo.
In fact, the IRP group has already begun utilizing differential tractography in its clinical trial. A mid-trial analysis2 of the data, which is currently under peer-review pending publication in a scientific journal, shows promising results — results that surely arrived more quickly thanks to the unique research environment at NIH, which gave Dr. Acosta’s team the freedom to try out approaches like differential tractography when the payoff was far from guaranteed.
“The IRP provides the possibility of exploring new techniques,” Dr. Acosta says. “When we start working with new techniques, there are a lot of unknowns. We didn’t explore only differential tractography — we have also explored three or four other MRI techniques, and this is one that worked very well. There is a lot of trial and error here. If we think there’s something that has potential, we can stick with it and make two or three attempts, whereas at other institutions you would just give up and say it’s not worth it anymore.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Lewis CJ, Vardar Z, Kühn AL, Johnston JM, D'Souza P, Yousef MH, Gahl WA, Shazeeb MS, Tifft CJ, Acosta MT. Differential tractography: an imaging marker for tissue degeneration in neurodegenerative diseases. Brain Commun. 2025 May 24;7(3):fcaf198. doi: 10.1093/braincomms/fcaf198.
[2] Lewis CJ, D'Souza P, Johnston JM, Acosta MT, Farmer C, Baker EH, Crowell A, Mojica Y, Rahman S, Joseph L, Hartman A, Vézina G, Quezado Z, Yousef MH, Luckett A, Vardar Z, Shazeeb MS, Corti M, Blackwood M, Coleman K, Thurm A, De Boever E, Gahl WA, Byrne BJ, Flotte TR, Jiang X, Gross AL, Keeler AM, Gray-Edwards H, Martin DR, Sena-Esteves M, Tifft CJ. AAV9 Gene Therapy in GM1 Gangliosidosis Type II: A Phase 1/2 Trial. medRxiv [Preprint]. 2025 Jul 29:2025.07.28.25332074. doi: 10.1101/2025.07.28.25332074.
Related Blog Posts
This page was last updated on Tuesday, September 16, 2025
