Picturing Stroke Recovery
IRP’s Larry Latour Peeks Into the Damaged Brain
IRP researchers are using a variety of advanced imaging techniques to learn more about what strokes do to the brain and how brains recover from those effects.
The word ‘stroke,’ attributed to the idea of ‘a stroke of God’s hand,’ was first used in 1599 to describe the sudden seizure, paralysis, and brain damage that was previously called ‘apoplexy.’ It was a fitting analogy. Strokes, which affect nearly 800,000 Americans every year, hit suddenly and terrifyingly, with devastating consequences. Speed is critical to good treatment outcomes, but until recently very little could be done.
May is Stroke Awareness Month, a time to draw attention to the risks and symptoms of stroke and the new treatments that are helping people recover with fewer lasting effects. We recently spoke with IRP senior scientist Lawrence L. Latour, Ph.D., an expert on brain imaging who leads the Acute Cerebrovascular Diagnostics Unit, a unique partnership between the NIH Intramural Research Program and two hospitals in the metro, D.C., area: Suburban Hospital and Medstar Washington Hospital Center. The collaboration, launched in 2000, aimed to incorporate magnetic resonance imaging (MRI) in examinations of patients experiencing symptoms of stroke. This allowed the clinicians to diagnose patients more easily and then, through imaging at later time points, look at how patients responded to their treatments in order to learn ways to improve therapy.
The effects of strokes vary widely. In most cases, they are caused by a blockage in the blood vessels of the brain, which can range from very mild disturbances to massive occlusions that can kill or permanently disable the person. One focus of Dr. Latour’s research involves people with these very severe strokes.

Dr. Lawrence “Larry” Latour
Until recently, the most seriously affected stroke patients had few treatment options. Although tissue plasminogen activator (tPA), the drug that breaks up stroke-inducing blood clots, was a breakthrough when it was introduced in 1995, it had significant drawbacks. Overall, for the benefit from tPA to outweigh the risks, patients need to be treated within 4.5 hours of symptom onset. Today, doctors can also use a newer therapy called mechanical thrombectomy, in which doctors carefully thread a device through the body’s arteries and into the brain’s to pull the clot out. Several different types of devices are used, from mesh contraptions that ensnare clots to suction devices that vacuum them up, and all together the various approaches can successfully remove the stroke-inducing blood clot in more than 80 percent of patients.
“Mechanical thrombectomy has changed everything,” Dr. Latour says. “Many stroke patients who once would have died or been permanently disabled for life are now walking out of the hospital in days with very little damage. It’s miraculous.”
Although continued research has allowed doctors to use this therapy for more patients, with impressive results, there are still many patients who don’t recover afterwards. Dr. Latour and his collaborators are trying to find out why. He believes taking MRI images right after the mechanical thrombectomy is the key to figuring this out.
“It’s been surprising, what we’re seeing,” he says. “It’s not what we would have expected based on the images we saw in patients who were treated with tPA, possibly because of the speed with which blood flow to the brain is restored.”
The advanced MRI scanners that Dr. Latour and other IRP researchers use in their studies can be so big they take up an entire room.
On the positive side, he explains, they saw that some of the most damaged tissue began to recover after the clot was removed. 1 Although this healing is rare, Dr. Latour believes it may indicate that removing the clot early enough helps spare more brain tissue from damage. On the other hand, the treatment can cause the newly unblocked bloodstream to push smaller clots further along, where they can still do damage. And, in some cases, the blood vessel remains blocked.2
“We’re trying to understand why that’s happening and if there’s a treatment for that,” Dr. Latour says.
His team has also discovered that when the blockage is successfully removed, blood flow to the affected region can double. That can be a good thing, because it means blood is providing nutrients and oxygen to cells again, but it can also lead to swelling and bleeding.3
“Doctors are not going to get a whole lot better at getting the clots out or getting to the patients earlier at this point,” Dr. Latour says, “so we need other therapies that can help treat the secondary damage. And that’s, again, where imaging may help.”
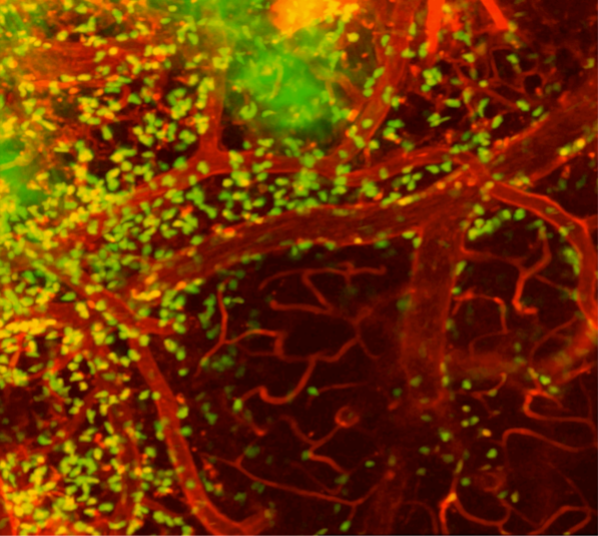
Dr. Latour also studies how immune cells in the brain respond to a stroke. This image from his experiments shows inflammation-inducing immune cells (green) swarming around blood vessels in the brain of a mouse model designed to mimic human strokes.
Meanwhile, Dr. Latour and fellow IRP senior investigators Dorian McGavern, Ph.D. and Joseph A. Frank, M.D., are also investigating the biology of stroke damage and repair using a mouse model they created. In one experiment, they injected tiny bubbles into mice to simulate the damage a stroke does to blood vessels in the brain. Then, using a technique called two-photon microscopy, they watched immune cells move around and attack or repair affected tissue. They discovered that tiny immune cells in the brain called microglia move in to seal off the damaged section of blood vessels, but if that doesn’t work, other immune cells can trigger dangerous inflammation or swelling as fluid builds up in the brain. The researchers found that a treatment that blocked those trouble-making immune cells prevented severe swelling but also interfered with the repair work of the microglia.4
“Understanding this process gave us hope there could be a way forward to stop this rapid swelling from happening,” Dr. Latour says.
Dr. Latour hopes these findings will ultimately improve life for people experiencing strokes, and he credits those patients for their contribution to his team’s studies.
“We find huge community support for the NIH and the work we do,” he says. “It's humbling. Our patients put a lot of faith and trust in us, and they’re giving us valuable time at a particularly stressful point in their lives. We need to make sure their contribution to science and to our research is really appreciated.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Hsia AW, Luby M, Cullison K, Burton S, Armonda R, Liu AH, Leigh R, Nadareishvili Z, Benson RT, Lynch JK, Latour LL. Rapid Apparent Diffusion Coefficient Evolution After Early Revascularization. Stroke. 2019; 50(8):2086-2092. Epub 2019 Jun 26. doi: 10.1161/STROKEAHA.119.025784.
[2] Luby M, Merino JG, Davis R, Ansari S, Fisher M, Hsia AW, Kim Y, Latour LL, McCreedy ES, Sukhdeo Singh R, Wright CB, Lynch JK. Association of Multiple Passes during Mechanical Thrombectomy with Incomplete Reperfusion and Lesion Growth. Cerebrovasc Dis. 2022;51(3):394-402. doi: 10.1159/000519796.
[3] Luby M, Hsia AW, Lomahan CA, Davis R, Burton S, Kim Y, Craft V, Uche V, Cabatbat R, Adil MM, Thomas LC, De Vis JB, Afzal MM, McGavern D, Lynch JK, Leigh R, Latour LL. Post-ischemic hyperemia following endovascular therapy for acute stroke is associated with lesion growth. J Cereb Blood Flow Metab. 2023 Jun;43(6):856-868. doi: 10.1177/0271678X231155222.
[4] Mastorakos P, Mihelson N, Luby M, Burks SR, Johnson K, Hsia AW, Witko J, Frank JA, Latour L, McGavern DB. Temporally distinct myeloid cell responses mediate damage and repair after cerebrovascular injury. Nat Neurosci. 2021; 24(2):245-258. Epub 2021 Jan 18. doi: 10.1038/s41593-020-00773-6.
Related Blog Posts
This page was last updated on Wednesday, May 29, 2024
