Hunger Hormone Feeds Alcohol Cravings
IRP Researchers Pursue New Approaches to Treating Alcohol Use Disorder

A hormone called ghrelin that regulates hunger appears to also influence cravings for alcohol, according to research by IRP investigators pursuing new treatments for alcohol use disorder (AUD).
While most adults in the U.S. consume alcohol in moderation, for nearly 30 million of them, going even one day without alcohol feels nearly impossible. For these Americans, alcohol use disorder (AUD) is a serious condition that harms their health, relationships, and career. Unfortunately, only a small percentage of people with AUD receive treatment, and even then, for many patients, the chances of a relapse are high.
As the search for a reliable and effective treatment continues, IRP senior investigator Lorenzo Leggio, M.D., Ph.D., is exploring the biological processes that underlie alcohol cravings to unlock new approaches to therapy. April is Alcohol Awareness Month, so we took the opportunity to speak with him about recent discoveries made by his IRP team and its collaborators.
“Addiction is a chronic medical disorder with quite complex mechanisms that really involves the brain and the whole body,” Dr. Leggio says.
One of the brain-body connections Dr. Leggio’s team is studying involves a hormone made primarily in the stomach called ghrelin. While ghrelin is best known for its role in regulating hunger, previous animal studies have shown it also plays a role in alcohol cravings. In 2017, to determine if this was also the case in humans, Dr. Leggio and his colleagues, IRP senior investigator Vijay Ramchandani, Ph.D., and staff scientist Reza Momenan, Ph.D., conducted an experiment in patients with AUD to see if raising their ghrelin levels would spur them to use more alcohol. 1 The study's participants received either intravenous infusions of ghrelin or a placebo during four different visits to the NIH Clinical Center. They also had a second intravenous line delivering small doses of alcohol directly into their blood, which they could control themselves. Sure enough, participants administered more alcohol to themselves during the study sessions when their ghrelin levels were elevated than when they received the placebo.
What’s more, repeating the experiment while the participants underwent functional magnetic resonance imaging (fMRI) brain scans suggested a biological reason for that result. When participants in the fMRI scanner viewed alcohol-related images, the ghrelin infusions boosted activity in a different reward-related network of brain regions compared to when the participants saw images related to food. When they saw the food images, a brain region called the nucleus accumbens, which is associated with the reward-related brain chemical dopamine, lit up when ghrelin levels were elevated. On the other hand, when they viewed alcohol-related images, ghrelin lit up the amygdala, a brain structure that is important for emotions and stress and plays a key role in AUD.
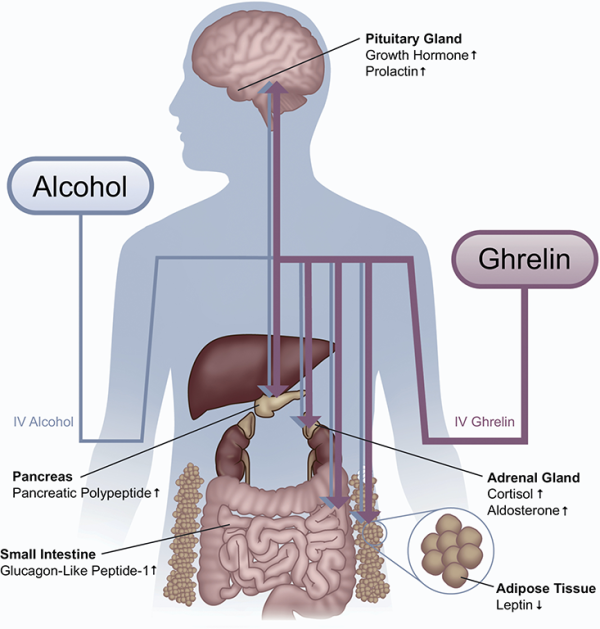
This diagram shows the wide-ranging effects that alcohol and ghrelin have on the production of various hormones by the body and brain, including the stress hormone cortisol.
“We were actually surprised to see this sort of differentiation — that the brain was reacting to ghrelin in a different way based on whether there was exposure to alcohol cues versus food cues,” Dr. Leggio says. “Adrenaline and cortisol are part of something called the hypothalamus-pituitary-adrenal axis, and we know ghrelin activates that system, but in people with AUD, that system is kind of blunted and less responsive to acute stress. What we discovered is that ghrelin was actually waking up that system.”
With this knowledge in hand, Dr. Leggio and his research team looked for ways to reduce alcohol cravings by blocking ghrelin’s effects. This proved to be a challenge, as there were no compounds used in clinical practice that could be re-purposed for use as ‘ghrelin receptor antagonists,’ meaning they could block the effects of ghrelin in the brain. Luckily, a new NIH grant opportunity came up just at the right time. The new program allowed IRP investigators to team up with pharmaceutical companies to develop new medications, which NIH would then test.
“When this grant was announced, I saw that the pharmaceutical company Pfizer had a ghrelin receptor antagonist,” Dr. Leggio says. “The wow-factor was that this was the only ghrelin antagonist in the world that a pharmaceutical company had already tested in humans. It was an offer you couldn’t refuse.”
Dr. Leggio and a colleague at the University of Rhode Island applied for the grant together and began testing the compound for safety and efficacy, first in animals and then in humans. 2 For the human studies, Dr. Leggio’s team took advantage of the NIH Clinical Center’s Bar Lab, which he constructed when he came to NIH in 2012. The room allows IRP researchers to conduct studies with human subjects in an environment resembling a typical bar, complete with bar stools, beer taps, and a dark, intimate atmosphere, but under well-controlled conditions and with proper medical oversight.

Some of Dr. Leggio’s studies take advantage of the NIH Clinical Center’s Bar Lab, pictured here.
“While our focus was on the safety of the ghrelin receptor antagonist, nonetheless, we also observed that people in the Bar Lab craved less alcohol and were less attentive to alcohol triggers when they took the drug.”
Now, Dr. Leggio’s team is working to find additional ways to interrupt the signals ghrelin sends to the brain before they even start. For example, before ghrelin can interact with its receptor, it must undergo a structural change that requires the help of an enzyme called ghrelin O-acyltransferase, or GOAT, in a process that he likens to starting a car.
“Ghrelin is the accelerator, and the receptor is the engine,” he explains. “If you want to push the accelerator and start the engine, you first need to turn it on with a key – that’s what GOAT is. So, the question is, can we treat addiction not by blocking the engine but by holding back the key? This is the initial clinical study we are running now.”
It will likely be years before a ghrelin-targeting therapy for AUD hits the market, as safety and efficacy will need to be investigated in larger studies. However, the long timeline does not phase Dr. Leggio. Though developing new treatments for AUD is challenging, he has been heartened by the rewarding relationships he has developed with other researchers both inside and outside the IRP.

Dr. Lorenzo Leggio
“Collaborative work always ends up being better than if you try to work in isolation,” he says. “Our growing story about ghrelin is a testament to that. We have built a complex orchestra to play this opera, ‘Ghrelin and Addiction,’ with the Clinical Center and IRP laboratories in multiple NIH Institutes and Centers, outside academic research centers, and industry, all working together to move forward our understanding of the ghrelin system in the context of addiction.”
“The goal and mission of my lab, and my own passion as a physician and scientist, is to develop new treatments for our patients with addiction and improve people’s lives,” he adds. “This challenge is an opportunity for excitement. Medication development is something close to my heart and I really dedicate my professional life to the mission.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Farokhnia M, Grodin EN, Lee MR, Oot EN, Blackburn AN, Stangl BL, Schwandt ML, Farinelli LA, Momenan R, Ramchandani VA, Leggio L. Exogenous ghrelin administration increases alcohol self-administration and modulates brain functional activity in heavy-drinking alcohol-dependent individuals. Mol Psychiatry. 2018;23(10):2029-2038.
[2] Lee MR, Tapocik JD, Ghareeb M, Schwandt ML, Dias AA, Le AN, Cobbina E, Farinelli LA, Bouhlal S, Farokhnia M, Heilig M, Akhlaghi F, Leggio L. The novel ghrelin receptor inverse agonist PF-5190457 administered with alcohol: preclinical safety experiments and a phase 1b human laboratory study. Mol Psychiatry. 2020;25(2):461-475.
Related Blog Posts
This page was last updated on Wednesday, May 24, 2023
