Illuminating the Brain’s Hidden Secrets
New Molecules for PET Scans Shed Light on Psychiatric Disorders
IRP researchers are developing new chemicals that can help neuroscientists investigate the roots of psychiatric disorders like depression using positron emission tomography (PET) scans.
For most kids, witnessing a classmate get teased for strange behavior or learning struggles is an unfortunate but common part of life. Growing up, IRP senior investigator Robert Innis, M.D., Ph.D., viewed the situation differently when he observed it during a high school geometry class.
“Some of my classmates were criticizing one student like he was a bad person,” he remembers. “I kept thinking, ‘You can’t blame him. It’s the chemistry in his brain that causes him to act that way. Don’t think you’re so high and mighty. It’s just your chemistry!’”
That interest in brain chemistry and how it relates to normal and abnormal behavior laid the foundation for Dr. Innis’s research in neuropsychiatry and brain imaging. As we observe World Brain Day on July 22, we took the opportunity to talk with Dr. Innis about his research, which uses a technique called positron emission tomography (PET) to measure the levels of various proteins in people’s brains and learn about their function in both healthy states and neuropsychiatric diseases.
A PET scan is a powerful imaging tool that can show biochemical activity in the body. It works by introducing a man-made ‘radioligand’ into the body, which consists of two parts: a ‘ligand’ that binds only to a specific protein of interest and a radioactive atom called a ‘tracer’ that the PET scanner can detect. When a person undergoes a PET scan after being injected with a radioligand, the resulting images of the body or brain will appear brighter in places where there are higher levels of the protein the radioligand is designed to bind to.
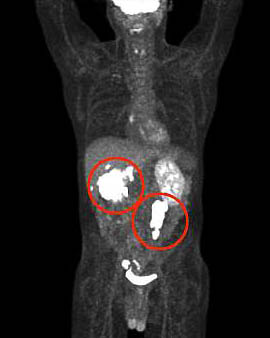
PET scans can reveal the location and concentration of any protein scientists or doctors are interested in as long as they use the appropriate radioligand. This PET scan shows the location of tumors in a patient’s body.
Dr. Innis’s work centers around creating new radioligands for use in diagnosing and understanding diseases and testing how well new therapies work. Over the years, he has created dozens of new radioligands, including one that is now used to diagnose Parkinson’s disease. Recent studies from his laboratory have focused on using PET imaging to detect inflammation in the brain, which can help researchers investigate mental health conditions such as depression, alcohol use disorder, and dementia.
“We’re further behind in our understanding of mental health conditions than neurological ones because we don’t have definitions of the disorders based on brain abnormalities,” Dr. Innis says. “For example, depression has multiple causes, multiple symptoms, and multiple possible treatments. There are probably about 20 genes that increase the chances of developing it. It’s not a specific diagnosis and there are no biomarkers, so instead, we’re studying how certain proteins in the brain might be different in people with depression.”
In one series of studies, Dr. Innis and his team created two radioligands that target markers of inflammation in the brain, COX-1 and COX-2, and then tested their ability to measure inflammation. Past research had suggested that, for some patients, depression might be caused by or somehow related to inflammation in the brain, and Dr. Innis wondered whether an anti-inflammatory drug might help alleviate symptoms in those patients.
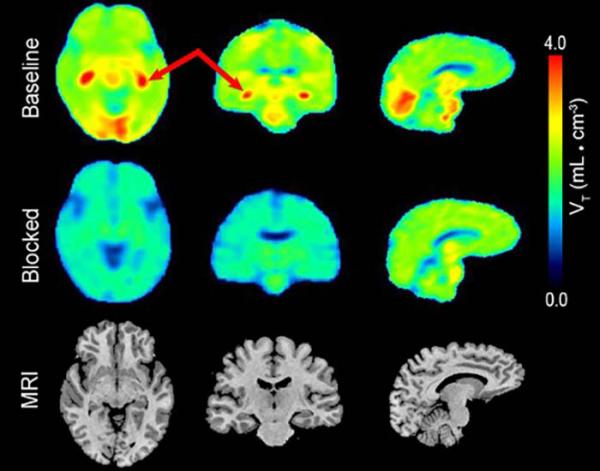
These PET images, taken using Dr. Innis’s COX-1-targeting radioligand, show where COX-1 is most highly concentrated in the brain of a healthy human participant (first row). Levels of that inflammatory marker fell dramatically when the participant was treated with a COX-1 inhibitor (second row).
Those new COX-1 and COX-2 radioligands have given researchers two valuable tools with which to test that idea, and Dr. Innis and his collaborators are planning to use them to explore the effects of inflammation in a variety of disorders, including depression, long-COVID, and Alzheimer’s disease.
“There’s light at the end of the tunnel that PET imaging of the brain could identify subgroups of patients who would be more likely to respond to certain novel treatments,” Dr. Innis says.
Another promising use of PET imaging is related to understanding and possibly treating alcohol use disorder (AUD). Strong evidence reaching back to the 1970s suggests that high alcohol consumption increases the activity of a signaling molecule called cyclic adenosine monophosphate (cAMP) in most areas of the brain. In addition, studies found that stimulating cAMP activity reduces alcohol consumption in animal models of AUD. Working in collaboration with fellow IRP senior investigators Nora D. Volkow, M.D. and George F. Koob, Ph.D., Dr. Innis created a radioligand that binds to a chemical called phosphodiesterase-4 (PDE4), which is a marker for cAMP activity. PET studies from his laboratory found that a subtype of PDE4 called PDE4B could be measured in alcohol-consuming rats using the radioligand.1 The rats initially showed increased binding of the radioligand to PDE4, indicating higher cAMP activity; however, if the rats became addicted to alcohol, their bodies adapted, and cAMP activity dropped. When the researchers kept the rats sober for two weeks, cAMP activity returned to normal levels. They now plan to do the same experiment in humans, first with social drinkers and then with people being treated for AUD.
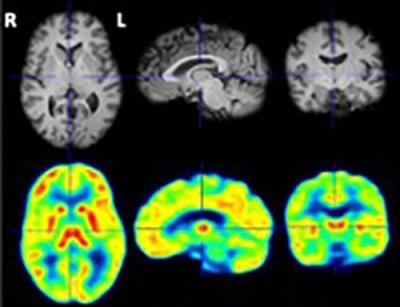
PET image (bottom row) of PDE4B in the brain of a healthy human volunteer, as detected using Dr. Innis’s PDE4-binding radioligand.
“We’re predicting that these patients will have decreased cAMP when they first come in and then, when they leave 28 days later, it should be normalized again,” Dr. Innis says.
Interestingly, PDE4B has also been implicated in depression. Ongoing studies in Dr. Innis’s laboratory are investigating whether PDE4B levels are higher in people with depression and whether a relationship exists between PDE4B levels and the severity patients' symptoms. Ultimately, this work will help establish whether PDE4B can be used as a biomarker for depression as well as other conditions related to inflammation in the brain.
While such studies provide important insights into the origins of neuropsychiatric diseases, the tools and expertise to do the work are very expensive and not widely available. Most institutions pay little attention to funding the development of imaging technology. The NIH, however, has three cyclotrons — machines that make radioactive atoms — as well as multiple PET scanners and a wealth of experts in subjects like radiochemistry. These resources have allowed Dr. Innis to take a leading role in his field of research.

Dr. Robert Innis
“I am a toolmaker — my lab makes these tools, and we try to share them with external research sites where this work would be difficult to get funded,” Dr. Innis says.
In this way, Dr. Innis and NIH contribute to our understanding of these illnesses in multiple ways: by investing in the tools needed to advance PET research, by conducting cutting-edge PET studies, and by working hard to share their results with scientists worldwide who can benefit from both the tools and the research findings.
“It fulfills a lifelong ambition to understand the chemistry of the brain, and I think that it will lead the way to specific and improved treatments,” Dr. Innis says. “That's my major motivation, and NIH is the ideal place to do it.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Olsen-Dufour A, Tang S, Innis R, Jenkins M, Lee A, Person T, Liow J-S, Kim S, Wu S, Freaney M, Vendruscolo L, Vendruscolo J, Morse C, Ahmadi P, Zoghbi S, Pike V, Zanotti-Fregonara P, Koob G, Volkow N. Radioligand binding to phosphodiesterase-4B as an indirect measure of cAMP cascade in a rat model of alcohol use disorder. Biological Psychiatry. May 1, 2023; 93(9):S130. doi: 10.1016/j.biopsych.2023.02.330.
Related Blog Posts
This page was last updated on Monday, July 22, 2024
