Gene Therapy Protects Neurons From Alzheimer’s Disease
New Approach Preserves Cognitive Abilities in Pre-Symptomatic Mice
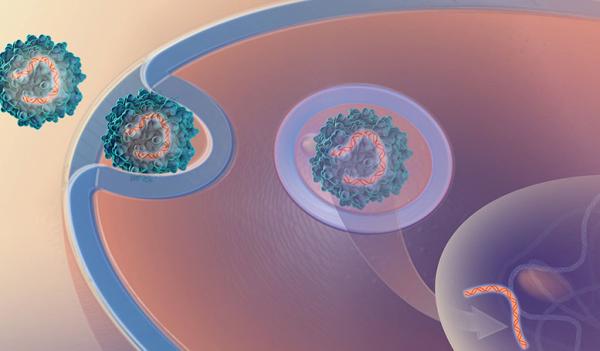
IRP researchers recently tested a gene therapy that uses a modified virus to deliver a gene into neurons that helps them survive the ravages of Alzheimer’s disease.
Cooks preparing traditional holiday feasts will surely find that a fire extinguisher can effectively quench an oven inferno, but it would probably be better if the oven never caught fire in the first place. Similarly, Alzheimer’s researchers have focused much more on putting out the biological fires scorching patients’ brains than on making brain cells ‘fireproof.’ A new IRP study in mice, however, suggests better results might be achieved with an approach specifically designed to make patients’ neurons more resilient.1
Alzheimer’s disease is marked by the build-up of ‘plaques’ in the brain made up of malformed amyloid-beta protein, as well as tangled masses of a protein called tau. Most research on Alzheimer’s has focused on those prominent signs of the disease, but treatments designed to erase them have so far yielded underwhelming results.
Scientists in the lab of IRP senior investigator Yoke Peng Loh, Ph.D., instead aim to treat the illness by helping neurons survive the onslaught of those plaques and tangles. They hope to do so by increasing the amount of a molecule called neurotrophic factor alpha-1 (NF-alpha-1) in the cells. This substance acts like a cellular flame retardant, influencing several cellular systems to keep neurons intact when they would otherwise die.
“This treatment is a multi-factorial approach,” Dr. Loh explains. “Taking an approach with multiple effects is, we believe, the best way to stop the cells from dying. It’s like having a rescue squad with firefighters and ambulances coming from every direction, as opposed to the approaches many drug companies have taken that focus only on clearing plaques.”
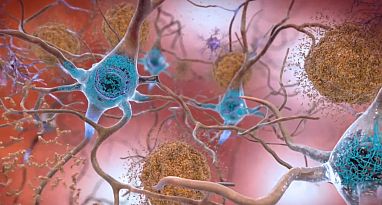
The brains of Alzheimer’s patients contain amyloid plaques (blue) and tau tangles (brown), but treatments focused on eliminating them have so far provided minimal benefits to patients.
In the new study, Dr. Loh’s team — led by the study’s first author, Lan Xiao, M.D., Ph.D. — tested a gene therapy that uses a modified virus to give neurons extra copies of the gene that produces NF-alpha-1. The researchers gave the therapy to male mice that have three of the same genetic mutations that predispose humans to developing Alzheimer’s disease. Without treatment, those mice reliably develop “full-blown Alzheimer’s symptoms” by six months of age, Dr. Loh says. The team used only male mice for this proof-of-concept experiment because their behavior tends to be more consistent than female mice, which have levels of reproductive hormones that fluctuate over time.
Dr. Loh’s team injected the gene therapy directly into the hippocampus, a part of the brain critical for memory, when the mice were two months old and had not yet begun showing any signs of Alzheimer’s disease. Not only did the gene therapy successfully boost levels of NF-alpha-1 by 50 to 80 percent in the hippocampus, but five months after being given the treatment, the mice that received it also did significantly better on a learning and memory test called the Morris Water Maze. This task requires mice to learn and remember the location of a platform in a pool of water. Healthy mice spend less time in the water with repeated practice on the task because they find the platform faster.
“This is a learning process,” Dr. Xiao explains. “Some mice, if they memorize well, will swim straight to the platform because they know it’s there.”
However, after the mouse has learned the location of the platform, the platform is removed. Mice with better memory will still swim towards where the platform used to be and spend more time in that area than mice with poor memory — and that was exactly the case when the IRP researchers compared the mice given the gene therapy to untreated mice.
When the IRP researchers looked at the animals’ brains, they discovered a litany of reasons why the treated mice retained their learning and memory capabilities while the untreated mice did not. For example, the gene therapy helped more neurons survive in the hippocampus, and the brains of mice given the gene therapy ended up harboring less of the precursors to the tau tangles and amyloid-beta plaques so closely associated with Alzheimer’s. The gene therapy also tamped down inflammation in the hippocampus, at least in part by decreasing the activity of immune cells called microglia that cause inflammation.
In addition, the gene therapy had positive influences on neurons’ energy-producing mitochondria. Mitochondria in neurons from the treated mice had higher levels of a molecule called Bcl2 that helps keep mitochondria alive and lower levels of a substance called Bax that destroys mitochondria. Neurons from treated mice also had lower levels of a molecule called Plin4, which suppresses a process known as mitophagy that gets rid of dead or malfunctioning mitochondria. Higher levels of Plin4 in the brain have previously been associated with Parkinson’s and Alzheimer’s disease.
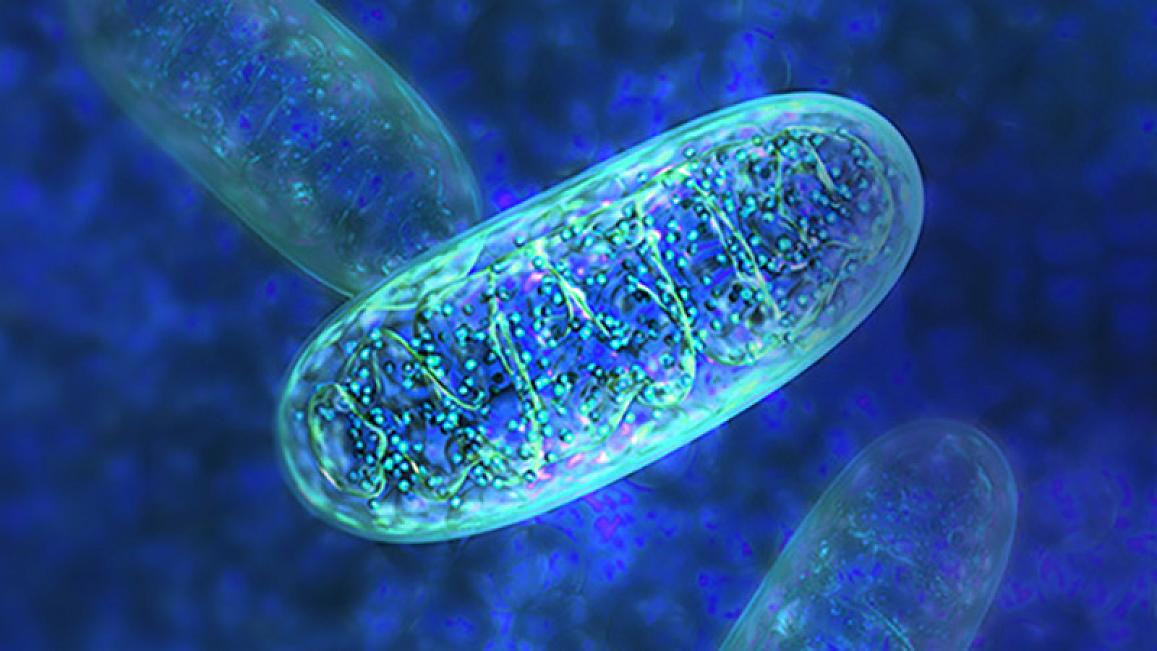
Part of the gene therapy’s benefits stemmed from its effects on cells’ rod-shaped mitochondria, which produce the energy that powers cells.
"If you have a lot of dead mitochondria and you don't remove them, you're going to have problems,” Dr. Loh says. “What our treatment does is to bring production of that mitophagy inhibitor down so cells can get rid of bad mitochondria.”
As promising as the study’s results are, it was still only a proof-of-concept experiment in mice treated before they had begun showing any symptoms of Alzheimer’s. As such, in addition to refining the delivery method for the treatment, Dr. Loh’s team plans to test whether it can also help mice that have already begun showing symptoms.

Dr. Yoke Peng Loh
At this point, boosting levels of NF-alpha-1 appears to hold promise for slowing the course of Alzheimer’s if it is done before the disease has caused much damage. As scientists continue to study the illness and discover ways to diagnose it early, more and more people at risk of Alzheimer’s may be identified early enough to gain some benefit from a treatment like the one developed by Dr. Loh’s team, even if the therapy cannot completely halt the disease’s effects.
“Alzheimer’s disease is a progressive disease, and so far there’s no effective strategy to completely stop it or recover the lost neurons,” says Dr. Xiao. “The only strategy is early diagnosis and early treatment that can slow progression of the disease.”
“It would be great if we can just keep patients at a stage where they can still function on their own versus real end-stage Alzheimer’s disease where they have to go into special care,” Dr. Loh adds. “If they can retain good enough cognitive function where they can live and manage daily life without endangering themselves, it would be much better.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Xiao L, Yang X, Sharma VK, Abebe D, Loh YP. Hippocampal delivery of neurotrophic factor-α1/carboxypeptidase E gene prevents neurodegeneration, amyloidosis, memory loss in Alzheimer's Disease male mice. Mol Psychiatry. 2023 Aug;28(8):3332-3342. doi: 10.1038/s41380-023-02135-7.
Related Blog Posts
This page was last updated on Thursday, January 4, 2024
