Experimental Treatment Helps Neurons Recover From Damage
Mouse Study Could Lead to New Therapies for a Variety of Ailments
New IRP research shows that revving up a natural healing process helps neurons regrow the branch-like axons they use to send electrical signals to other cells.
In most parts of your brain, the set of neurons you’re born with is what you’ve got for life — just like your fingers and toes, if you lose any, they’re not coming back. The body does have ways to encourage healing after a brain injury, but they are extremely constrained. However, by lending those natural systems a helping hand, IRP researchers have managed to dramatically boost regeneration and recovery of vision in mice with damage to the nerves that connect the eyes to the brain,1 an approach that could one day help people recover from other types of nervous system injuries as well.
Neurons throughout our bodies send signals to one another and to our muscles via branch-like axons. Injuries to the spinal cord, the brain, or the ‘optic nerve’ that relays visual information to the brain can damage those axons. If the axons don’t recover, the neurons they extend from will soon die, leading to a decline in whatever their job was — whether vision, movement, planning, paying attention, or something else.
“You don’t want your neurons constantly propagating, so once they are made, they are stabilized, but the problem is they lose most of the function of proliferating,” explains IRP senior investigator Hee-Yong Kim, Ph.D., the new study’s senior author. “In skin and other body parts, when you are hurt, regeneration occurs — cells multiply and so on. Neurons don’t do that.”
“Axon regeneration after injury has been a problem for a long time and it’s still challenging,” she adds, “which is why we still have patients with spinal cord or optic nerve injuries who don’t recover.”
Unlike in most parts of the adult brain, when the brain is developing in-utero, new neurons need to be made and to extend their axons to create connections with other cells. One way they do this is through a receptor called GPR110, which spurs the birth of new neurons and the growth of axons when it is triggered by a chemical produced in the brain called synaptamide. Levels of GPR110 plummet after birth, but while studying a mouse model of traumatic brain injury, Dr. Kim’s team unexpectedly discovered that the injury caused the animals’ neurons to produce more of both the receptor and its synaptamide activator in order to promote healing.
“That’s a natural, spontaneous response,” Dr. Kim says. “Nevertheless, activating the receptor with synaptamide is not always very efficient in the situation of a massive injury.”
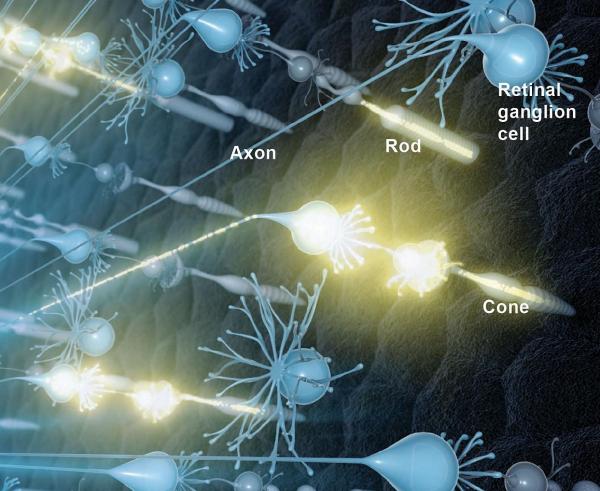
Light-sensing ‘rod’ and ‘cone’ cells in the eye relay visual information to retinal ganglion cells, which then pass it on to the brain via the optic nerve.
In the new study, a group of IRP researchers led by Dr. Kim investigated whether they could help the healing process along by injecting either synaptamide or a more potent, lab-designed form of the chemical, called A8, into the eyes of mice with injuries to the extra-long axons that make up the optic nerve. The researchers found that both treatments spurred axons in the optic nerve to regrow. As a result, treated mice had more axons in their previously injured optic nerves than untreated mice; however, treated mice lacking the gene for GPR110 did not. The two treatments also dampened the death of the cells in the eye, known as retinal ganglion cells, that transmit visual information from the eye to the brain via the optic nerve.
Of course, having intact axons after an injury is pointless if those axons don’t do their job, but Dr. Kim and her colleagues found that the animals given either the synaptamide or A8 treatment after the injury had more normal electrical responses in their brains to a visual cue, suggesting that their vision at least partially recovered. Once again, mice lacking the gene for GPR110 did not experience this improvement in visual function. Consistent with those results, regenerated axons in the treated mice connected to the correct target in the brain, called the lateral geniculate nucleus (LGN), and they also recovered their coating of a substance called myelin, which helps electrical signals travel along axons more quickly.
“The axons are in the right place, and that’s why the animal is having vision recovery,” Dr. Kim explains. “In a lot of cases, when you stimulate axon growth, they are going all over the place, meaning that they are not properly guided into the right place. However, this treatment appears to cause the injured axons to recover and extend to or remain in the right target.”

Dr. Hee-Yong Kim
The study’s results suggest that GPR110 activators might one day help people regain vision that has been degraded by an injury or a disease like glaucoma, in which high pressure in the eye causes the optic nerve to deteriorate. Such a treatment may also prove useful for people experiencing paralysis due to spinal cord injuries, since the spinal cord relays messages from the brain to muscles along axons just like the eye sends visual information to the brain along the optic nerve. As an added bonus, because only injured cells increase their levels of the GPR110 receptor, GPR110 activators may have fewer side effects than other approaches for regenerating damaged axons.
“The injured neurons that need the repair upregulate this receptor, so even if other cells are exposed to the receptor’s activator, they are not affected,” Dr. Kim explains. “That specificity is a great advantage of using this approach. This treatment only affects the cells that need it the most.”
“We see the potential,” she adds, “but in order to move to human clinical trials, we’ll need to team up with clinical researchers studying these axon injuries. We are actively looking for those opportunities right now.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Kwon HS, Kevala K, Qian H, Abu-Asab M, Patnaik S, Marugan J, Kim HY. Ligand-Induced Activation of GPR110 (ADGRF1) to Improve Visual Function Impaired by Optic Nerve Injury. Int J Mol Sci. 2023 Mar 10;24(6):5340. doi: 10.3390/ijms24065340.
Related Blog Posts
This page was last updated on Wednesday, September 13, 2023
