NIH Scientists Redesign Neurons to Enable Targeted Therapies
New Receptors and Radioactively Labeled Molecules Could Provide Useful Tools for Research and Medicine
Using ‘chemogenetic’ approaches, scientists like IRP investigator Mike Michaelides can control the firing of specific neurons in the brain in order to study their contributions to a wide range of neurological processes and illnesses.
Genetically modifying neurons to enable scientists and clinicians to influence brain activity probably sounds like the stuff of science fiction. However, the technology has existed for more than a decade, allowing scientists to make important leaps in understanding how neurons communicate with one another in healthy individuals and those with psychological and neurological conditions. What’s more, recent improvements to these tools developed by researchers led by IRP investigator Mike Michaelides, Ph.D., may allow neurologists to use them to deliver drugs to just the right brain cells to treat those ailments effectively without the side effects caused by current treatments.
To find their way through the maze of neurons and other cells that make up the brain, scientists use a technology called ‘chemogenetics’ that allows them to target only the specific neurons they want to manipulate. This allows them to modify cells so they can control and study specific neurons without invasive procedures like direct electrical stimulation. To accomplish this, they first encapsulate customized DNA sequences that code for lab-designed receptors in harmless viruses that can deliver that genetic material only to certain neurons, effectively tagging them for future activation or inhibition. Those selected cells will then start producing the receptor encoded by that DNA, which serves as a docking station for a specially created molecule, called a ligand, that can only interact with that receptor. In theory, because that ligand — essentially a ‘designer drug’ — can only bind to the lab-designed receptor, it can only affect the neurons that the scientists want to target. For that reason, the receptors are referred to as ‘DREADDs’, which stands for ‘Designer Receptors Exclusively Activated by Designer Drugs.’
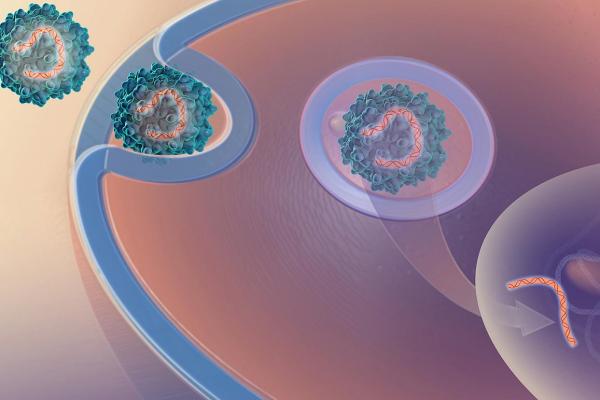
A virus introduces genetic material into a cell. Scientists take advantage of viruses' ability to do this in order to get their lab-designed DNA that codes for DREADD receptors into specific neurons.
DREADDs are currently used in laboratories around the world to study the brain circuitry behind a number of processes and disorders, such as Parkinson’s disease, pain, and substance use disorder. Researchers also hope that DREADDs will eventually prove useful for human therapies, but their utility is limited right now because the synthetic ligands that activate them can be fickle. They tend to form weak connections to the DREADD receptors on the tagged cells or even go off-course, interacting with receptors other than the DREADD. As a result, they may still cause side effects or fail to have any effect at all.
A team of IRP researchers and collaborators led by Dr. Michaelides, head of the Biobehavioral Imaging & Molecular Neuropsychopharmacology Unit at the NIH’s National Institute on Drug Abuse (NIDA), are fine-tuning the DREADD technique by creating new ligands that form stronger bonds with their target DREADD receptors, making their action more potent. In addition, Dr. Michaelides’ team created the first DREADD ‘radioligand,’ a ligand connected to a radioactive isotope that lights up in scans produced by an imaging technology called positron emission tomography (PET).1 By allowing scientists to track DREADD ligands in the body over time, this new radioligand will make it possible to monitor whether a designer drug hits the right target and whether a certain dose of it binds to enough receptors to induce the desired effect.
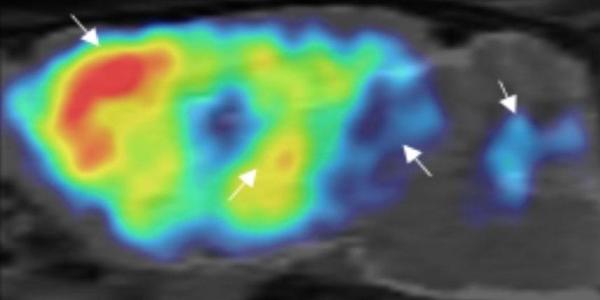
Side view of a mouse brain imaged non-invasively using PET and Dr. Michaelides’ radioligand to detect a specific DREADD. The arrows show where the DREADD is found most densely in the animal’s brain.
“Every time you introduce something foreign into the human body, you want to be able to visualize it,” Dr. Michaelides says. “If you want what you're injecting to go to a specific part of the body and function correctly, you want to know it’s in the right place. You would also be able to optimize the ligand dose because you could look at the interaction of the ligand with the receptor in real time.”
Moreover, the radioligand created by Dr. Michaelides’ team has an additional benefit: it takes longer to break down than other radioligands used in DREADD imaging. As a result, the radioligand can be produced at institutions that have the right facilities and then shipped to labs or clinics that lack that synthesis technology.
“You don't want to have patients flying to the few places that actually have the infrastructure to generate these radiochemicals,” Dr. Michaelides says. “Our radioligand can be transported from one place to another before it decays and is rendered useless.”

More From the IRP
Podcast
Dr. Peter Bandettini — Mr. MRI
Although DREADDs and other chemogenetic technologies are not being used in humans yet, scientists are studying several therapeutic applications for them. For instance, Dr. Michaelides and his team recently worked with a sleep specialist at The Johns Hopkins University to test whether the DREADD approach could be used to activate the tongue muscle in an animal model of sleep apnea. In people with sleep apnea, the tongue muscle relaxes and blocks the upper airway at the back of the mouth when they sleep, preventing them from breathing. With just a small injection similar to a novocaine shot from a dentist, the researchers caused the muscle to contract and open up the airway.

Dr. Mike Michaelides
Dr. Michaelides thinks the first applications of chemogenetic technology in humans will likely be for pain relief, so his lab is also gearing up to work with a biotech company to develop a chemogenetic approach that could alleviate the need for opioids and other medications to control pain. While still in pre-clinical development, such a therapy could become available in just a few years.
“There's a huge problem right now with effective pain medications,” Dr. Michaelides says. “Opioids work well at treating pain, but they have the potential for abuse and other side effects. We want to find a different way of relieving pain more precisely without liability for abuse and with fewer side effects.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References
[1] High-potency ligands for DREADD imaging and activation in rodents and monkeys. Bonaventura J, Eldridge MAG, Hu F, Gomez JL, Sanchez-Soto M, Abramyan AM, Lam S, Boehm MA, Ruiz C, Farrell MR, Moreno A, Faress IMG, Andersen N, Lin JY, Moaddel R, Morris PJ, Shi L, Sibley DR, Mahler SV, Nabavi S, Pomper MG, Bonci A, Horti AG, Richmond BJ, Michaelides M. Nat Commun. Oct 11 2019;10(1):4627.
Related Blog Posts
This page was last updated on Wednesday, May 24, 2023
