A Long Tradition of Vaccine Breakthroughs
IRP Vaccine Research Stretches Back to the NIH’s Birth
Over the past few months, the world has gained a new appreciation for the long, difficult process of producing vaccines as it waits anxiously for one that will provide protection from the novel coronavirus. With the NIH Vaccine Research Center’s efforts to develop a COVID19 vaccine drawing a huge amount of media attention, it is easy to forget that the IRP has been making vital contributions to vaccine development for more than 100 years. These efforts have helped produce vaccinations for smallpox, rubella, hepatitis A, whooping cough, human papillomavirus (HPV), and several other diseases. Read on for a visual journey through the history of IRP vaccine research.

A Young Agency Tackles Diptheria
In 1902, after a diphtheria outbreak in St. Louis, Congress gave the responsibility for regulating the safety and efficacy of vaccines to NIH’s precursor agency, the Hygienic Laboratory, established in 1887. NIH continued to provide oversight to commercial vaccine producers until the Food and Drug Administration (FDA) took over that function in 1972.
NIH Research Helps Eradicate a Deadly Virus

The smallpox vaccine, the first vaccine against any infectious disease, was also the first vaccine licensed (in 1903) by the Hygienic Laboratory. Over the next several decades, NIH scientists did important smallpox vaccine research, including Vernon Fuller and Robert Kolb’s work on standardizing a vaccine potency test in 1968. Smallpox was eradicated by 1980 through a vigorous global vaccination program. In this photo, U.S. Surgeon General William Stewart posed with Rebecca Ansah Asamoah, recipient of the 25-millionth smallpox vaccination.

This traveling exhibit in the NIH Stetten Museum’s collection demonstrated to physicians the many dermatological reactions people could have to smallpox vaccinations. The early 20th-century display was created for James Leake, an NIH epidemiologist who is best known for developing the multiple-pressure method for smallpox vaccination in collaboration with John Force. They used a flat blade pressed tangentially against the skin many times, a method that replaced scratching the skin. Over his long career, Dr. Leake set up a hospital for people who were ill with the 1918 influenza in Washington, D.C., and served as the chief of the Epidemiology Section at the NIH’s National Microbiological Institute, which was renamed the National Institute of Allergy and Infectious Diseases (NIAID) in 1955.
Combating a Lethal Tick-Borne Illness

This picture shows residents of Darby, Montana, lining up at a schoolhouse to get free vaccinations against Rocky Mountain spotted fever in the 1930s. The illness was spread by tick bites and was usually fatal.
Roscoe Spencer and Ralph Parker of NIH’s Rocky Mountain Laboratories in Hamilton, Montana, ground up infected ticks and mixed them with phenol to create a vaccine for the illness. Spencer vaccinated himself in 1924 and survived. Larger human trials showed that the vaccine provided complete protection in most cases. The vaccine became available in 1927, and by 1940 half a million people in the Rocky Mountain region had been inoculated with the Spencer-Parker vaccine. Nowadays, doctors treat Rocky Mountain spotted fever using antibiotics.

Investigating Approaches to Fighting Meningitis
In 1937, Sara Branham (right) and her technician Robert Forkish tested antiserum as a treatment for meningitis, an infection of the brain’s outer lining for which there was no vaccine at the time. Dr. Branham’s work included the taxonomy of the bacterial genus Neisseria and the description of new species in meningitis, which helped lead to a vaccine for the condition.
A New Way to Mass-Produce Vaccines

NIH researcher Herald Cox wasn’t preparing breakfast for a large party in this photo; he was inoculating egg yolk sacs with rickettsia organisms to create vaccines for typhus, Q fever, and Rocky Mountain spotted fever. Before he came up with this method in 1938, producing the vaccines was laborious, dangerous, and required the use of many laboratory animals. His method allowed NIH’s Rocky Mountain Laboratories to mass-produce needed vaccines during World War II. The Coxiellaceae family of organisms and the genus Coxiella, which contain the bacterium that causes Q fever, are named after Dr. Cox.

The First Tests for Vaccine Effectiveness
A vaccine is no good if it isn’t potent enough to provoke an immune response. The NIH developed tests for potency and standards for how potent vaccines had to be to work. For example, Margaret Pittman developed a way to test the potency of the vaccine for pertussis, also known as whooping cough. In 1949, along with Pearl Kindrick and Grace Eldering of the Michigan Department of Health, Dr. Pittman established the U.S. standards for the pertussis vaccine, which became the international standard. In this photo, Dr. Pittman (right) is showing the results of her test to NIH visitors.
Ensuring Vaccine Safety

In the mid-1950s, Ruth Kirschstein developed a safety test for the Sabin oral polio vaccine, which became the standard polio vaccine for decades. In 1957, Dr. Kirschstein became deputy director of the NIH’s Division of Biologics, which is now a part of the FDA and is known as the Center for Biologics Evaluation and Research. In 1974, she became the first female director of an NIH institute: the National Institute of General Medical Sciences. She served as NIH Deputy Director in the 1990s and as Acting NIH Director in 1993 and from 2000 to 2002. She is shown here using a Spencer Lens Co. Colony Counter, an instrument used to count colonies of bacteria or other microbes growing on a laboratory plate.

NIH Scientists Put Rubella on the Run
Following a major U.S. epidemic of rubella — also known as German measles — in 1964 and 1965, the U.S. Centers for Disease Control and Prevention (CDC) reported that at least 20,000 congenitally infected infants were born blind or deaf or with deformities, heart conditions, or intellectual disabilities. Tens of thousands of other parents lost their babies. The epidemic spurred NIH’s Harry M. Meyer Jr. and Paul D. Parkman to develop the rubella vaccine. Here Dr. Meyer (left) and Dr. Parkman (right), along with fellow NIAID scientist Hope Hopps, inspect a culture of the virus that causes rubella.
Making Hepatitis History

The NIH’s Robert Purcell (left) and Albert Kapikian (center) discovered hepatitis A virus particles using this microscope in 1973. The duo, along with the FDA’s Stephen Feinstone (right) and other collaborators, went on to develop the first vaccine for hepatitis A and discover hepatitis C.
With more than 10 million cases each year worldwide, hepatitis A is the most widespread of the viral hepatitis infections. Since hepatitis A vaccination became recommended in the United States in 1996, the number of reported cases in the country has dropped from roughly 31,000 per year to fewer than 1,500.
Banishing a Deadly Bacterium

Chances are that you’ve never heard of Hib, the nickname for Type B Haemophilus influenza, which causes bacterial meningitis. In the early 1980s, 20,000 children per year would get sick with Hib. Even with antibiotic treatment, 5 percent died and 30 percent suffered brain damage. Today, fewer than 100 people get Hib meningitis each year.
Hib has become another forgotten epidemic disease because of the work of NIH scientists John Robbins (left) and Rachel Schneerson (right), who helped develop the vaccine for Hib. In 1996, they won the Albert Lasker Award for Clinical Research along with Porter Anderson Jr. of the University of Rochester and David Smith of the David H. Smith Foundation. Here, Dr. Robbins poses with his Lasker Award.
IRP Vaccine Research Puts Pertussis in its Place
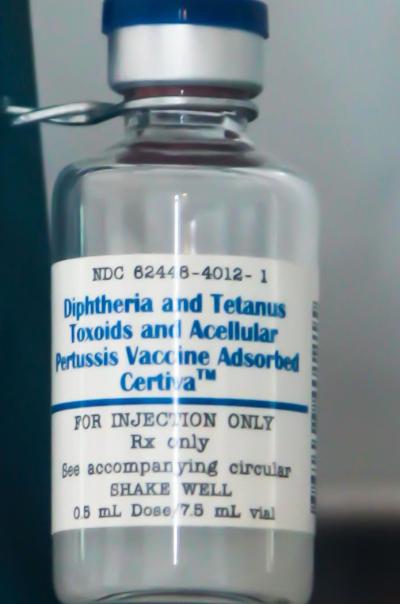
This vial of diphtheria, tetanus, and pertussis (DTP) vaccine from 1999 represents the long history that NIH has with each of those vaccines, beginning with Joseph Kinyoun, who founded the Hygienic Laboratory in 1887 and helped develop diptheria antitoxin, as well as set the standard for tetanus antitoxin in 1907. By the mid-1940s, vaccines for diphtheria, tetanus, and pertussis had been combined for delivery as the DTP vaccine.
In the early 1990s, a team of IRP researchers led by Ronald Sekura and John Robbins developed a new vaccine against pertussis. Their concept was based on Margaret Pittman’s theoretical work. Instead of whole, killed organisms, the vaccine contained only the parts of the pertussis bacterium important for triggering a protective immune response, resulting in fewer side effects. The NIH licensed it for commercial use in the late 1990s.
Two Decades of Work Culminates in a Cancer-Preventing Vaccine
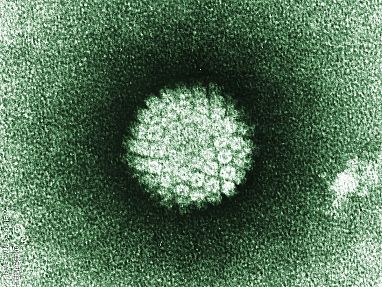
The human papillomavirus, or HPV, pictured here, is the world’s most common sexually transmitted infection and can cause several types of cancer. IRP researchers Douglas Lowy and John T. Schiller spent more than 20 years trying to figure out how to prevent HPV infection. They eventually found a way to produce virus-like particles (VLPs) that can stop HPV from infecting cells, leading to the first commercially available vaccine against the two deadliest forms of HPV in 2006.
The HPV vaccine has since been shown to be 100 percent effective, and governments across the globe now recommend routine vaccination of all girls (and in some countries, boys) aged 11 or 12 years. Widespread vaccination could reduce HPV-associated cancer deaths by up to two-thirds.
Developing vaccines for infectious diseases like COVID19 is a daunting challenge, but one that the NIH has been tackling since its inception. With its vast collection of cutting-edge resources and scientific expertise, as well as a tradition of interdisciplinary collaboration, the IRP is poised to continue making vital contributions to the prevention of communicable illness.
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
Related Blog Posts
This page was last updated on Tuesday, May 23, 2023
