The Nerve to Study CNS Regeneration at the IRP
I knew very little about neuroscience before beginning my graduate studies, but the topic of neurodegeneration looked very interesting. Having applied to several labs, I landed a Ph.D. student position in a neuroscience lab at the Saha Institute of Nuclear Physics, a government research institute in India, that would propel me on my way to the NIH IRP. In the first year of my Ph.D. program, I learned several things about the central nervous system (CNS), but what intrigued me the most was its lack of ability to regenerate after injury.
In the developing CNS, elaborate guidance cues specify which neuron connects with another neuron or non-neuronal cell. This complex process establishes the neural circuitry that will stay mostly unchanged throughout life. The mammalian CNS has evolved in such a way that once formed, it inhibits reconstruction of itself, resulting in partial or complete impairment of its functions after an injury.
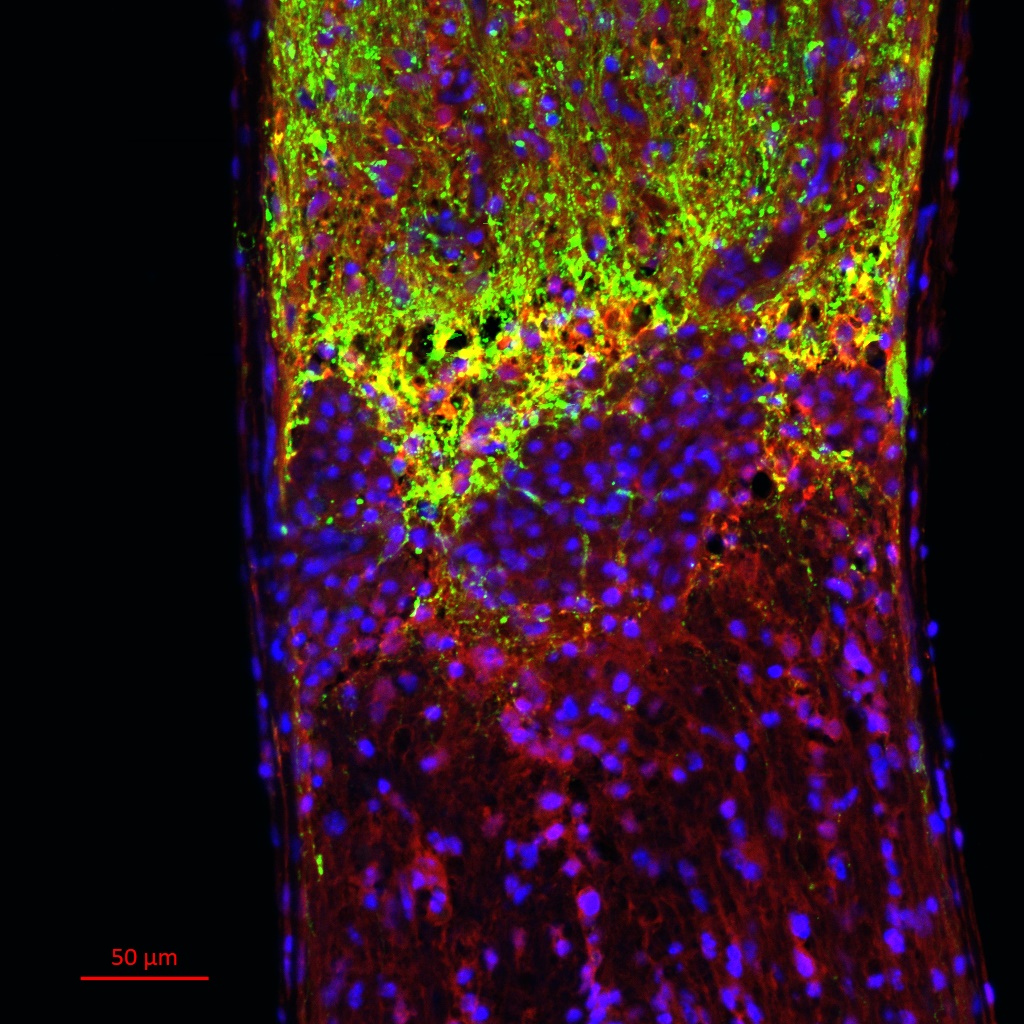
Retinal ganglion cell axons (green) fail to grow past the crush site in mouse optic nerve injury.
My doctoral research began by hunting down proteins in the cerebrospinal fluid associated with spinal cord injury (SCI), working closely with clinicians and patients. Seeing how patients’ quality of life drastically changed forever after severe SCI had a big impact on me. Apart from rehabilitative care, there is not much that we can do about reversing or improving SCI. By the time I completed my graduate studies, I was able to establish an understanding of the molecular cascade in the CNS following severe SCI.
Then, I made the journey from India to the United States and finally arrived at the NIH last summer, in the intramural program of the National Eye Institute (NEI). Finding a position of my choice wasn’t very straightforward, but I persisted, first by applying to labs that do research related to spinal cord regeneration (without luck), and then eventually broadening my search to labs studying CNS regeneration in general. After presenting my Ph.D. work and conversing with Dr. Tomarev, my current supervisor, and Dr. Nakaya, a staff scientist in our lab, I was accepted to the Section of Retinal Ganglion Cell Biology (SRGCB). I am now studying regeneration of the optic nerve after injury.

Building 6 at NIH, where I work
The human optic system is part of the CNS, and, like neurons in the spinal cord, retinal ganglion cells in the eye fail to regenerate past the injury site when the optic nerve is crushed. The molecular mechanisms that inhibit regeneration are similar in both cases, following the ‘blueprint of inhibition’ in CNS. My work revolves around investigating the efficacy of olfactomedin domain-containing proteins in facilitating optic nerve regeneration. I utilize a mouse model for this research.
Regeneration research at SRGCB is also part of a larger NIH effort: the NEI Audacious Goals Initiative. The primary aim of AGI is restoration of vision by retina regeneration, and it brings together vision researchers from around the world dedicated to regeneration research. It feels genuinely great to be a part of this endeavor. In parallel, I am also studying how the CNS ‘blueprint of inhibition’ has evolved differently in organisms that regenerate their CNS efficiently, like zebrafish. The zebrafish model provides a unique system to study regeneration, because of its capacity to regenerate its CNS into adulthood, unlike mammals. Moreover, the zebrafish model allows for fast and efficient genome editing. Knocking-in mammalian orthologs of regeneration-associated and regeneration-inhibitory genes in zebrafish could help us understand more about their mechanisms.
As I look back on the past year, I feel immensely lucky to be here and happy that I put in the effort required to find a position that fits my research interests. Every bit of trial, failure, and waiting was completely worth the rewarding research I’m now doing.
If you are also interested to learn more about CNS regeneration studies, the NIH IRP has several labs dedicated to this field, such as Drs. Leonardo Belluscio, Herbert Geller, Edward Giniger, and Henriette van Praag.
Related Blog Posts
This page was last updated on Monday, January 29, 2024
