Beauty Is in the Eye of the Gecko
NEI Researchers Use Geckos and Other Lizards to Accelerate Clinical Vision Research
The go-to lab animals to study many human eye diseases tend to be mammals—a mouse or a rat for example—because of their similar evolution and genetics compared to humans. But for diseases that affect a part of the eye called the fovea, which provides high-acuity central vision in humans, scientists are beginning to look further afield.

CREDIT: ASHLEY RASYS AND ANDREW WEGERSKI, NEI
Gecko eyes have features that mimic the human eye and are being used by Robert Hufnagel’s lab to study vision disorders. Shown: The gecko species Gonatodes antillensis.
Clinician-scientist Robert Hufnagel, Director of the National Eye Institute’s Ophthalmic Genomics Laboratory and Chief of the Medical Genetics and Ophthalmic Genomics Unit, thinks that lizards like the gecko (Gonatodes antillensis, Phelsuma lineata, Lygodactylus kimhowelli) can serve as a useful model to understand what’s happening in the eyes of his patients.
Hufnagel works with people who have rare genetic conditions that affect their vision. For these patients, few other people around the world have a similar disease, and in many cases, no animal models for their disease exists. This makes finding and testing treatments for their disease a challenge.

CREDIT: ROBERT HUFNAGEL, NEI
Robert Hufnagel believes that lizards like the gecko can serve as a useful model to understand what’s happening in the eyes of his patients.
“For rare diseases in humans, it can be really difficult to find enough patients with similar clinical diseases and genetic changes to translate those diseases to genotype:phenotype studies in an animal model. Being able to create relevant animal models to help study these rare diseases is important to our patients,” said Hufnagel. Genotype:phenotype studies reveal how individual sections of DNA physically manifest in the body.
Although he also works with mice (Mus musculus) and zebrafish (Danio rerio), Hufnagel has chosen the gecko—along with a close lizard relative, the anole (Anolis sagrei)—as his newest animal model. One of the reasons he chose the gecko is that the animal’s eyes have features that nicely mimic the human eye. Many of the most common mammalian animal models, like mice or rats, have what’s known as rod-dominant eyes, meaning they see mostly in black and white. They also tend to lack a thin part of the retina called the fovea, which helps humans have high-acuity central vision. The gecko, on the other hand, has a fovea, just like us.
“The geckos and anoles each bring their own attributes—there are advantages and limitations to working with each of these animals,” said Ashley Rasys, a postdoctoral fellow in Hufnagel’s lab.
The anoles, for instance, reproduce much more quickly and easily than the geckos. But the geckos, with their single fovea (compared with two foveas in the anole eye) are more similar to humans.

CREDIT: ASHLEY RASYS, NEI
Prior to coming to Hufnagel’s lab at NIH, Ashley Rasys developed and used the CRISPR technique to knock out the tyrosinase gene in the anole which leads to oculocutaneous albinism (OCA) in humans and other animals.
“By comparing both, we’ll be able to learn a lot about how the fovea develops and how mutations affecting certain genes can lead to foveal defects,” said Rasys. “There are a lot of similarities between the gecko’s eye structure and ours—it shows a very similar pattern of development to the human as well. Right now, we’re doing developmental staging on the gecko’s eye, and testing tools for genetic reprogramming.”
Hufnagel’s plan hinges on the CRISPR technology. This technique allows researchers to simply and quickly mutate a particular gene in an animal. Hufnagel plans to use this technique to match geckos to the genetic changes present in his patients. Then he can test novel treatments and therapies in the geckos, with a pretty good idea of what to expect from his patients.
Prior to coming to the NIH, Rasys developed and used the CRISPR technique to knock out the tyrosinase gene in the anole (Cell Rep 9:2288-2292, 2019). Loss of the gene leads to oculocutaneous albinism (OCA) in humans and other animals. In humans, OCA leads not only to reduced melanin pigment in the skin and eye, but also to developmental defects in the fovea that can cause low vision. “By knocking out tyrosinase and other genes in the lizard, we can evaluate an animal with abnormal development or degeneration of the fovea that resembles the human disease,” Hufnagel said.

CREDIT: ASHLEY RASYS AND ANDREW WEGERSKI, NEI
Geckos such as Phelsuma lineata have features that mimic the human eye, such as a fovea, and are being used by Hufnagel’s lab to study vision disorders.
By studying this disease in a species like the gecko, with eyes similar to human eyes, Hufnagel will be able to better understand what pathways are involved in foveal development.
“Not only do new tools like genome editing and genome assembly allow us to do these kinds of manipulations…, it accelerates our ability to choose an animal model based on the features of the organism, rather than the [models] that are already available. It gives us new ways to understand the particulars of certain diseases,” Hufnagel said. “As we see patients in clinic and find new genetic diseases, depending on the features we observe in the patient, we can pick the right [model] to study their disease.”
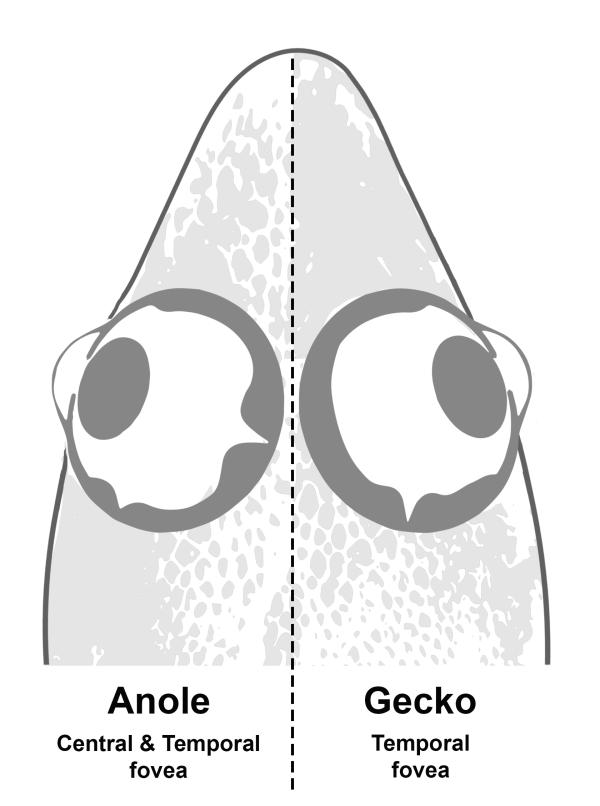
CREDIT: ASHLEY RASYS, NEI
A thin part of the retina called the fovea helps humans have high-acuity central vision. The eyes of the anole lizard and the gecko have fovea, just like us.
To connect with NIH researchers working with different types of animal models, consider joining the Interspecific Modeling Scientific Interest Group (https://oir.nih.gov/sigs/interspecific-modeling-interest-group).
Read about NIH-funded extramural researchers who are using fruit flies, newts, and zebrafish in their vision research at https://www.nei.nih.gov/about/news-and-events/news/small-creatures-teac….

Lesley Earl is a science writer and Section 508 coordinator in the Office of Science Communications, Public Liaison, and Education at the National Eye Institute. When not writing, she enjoys singing, growing vegetables, and meandering through the woods.
This page was last updated on Thursday, August 10, 2023
