Why Some Stay Sick
Unraveling Long COVID Alongside Other Post-viral Illnesses
Well before the term long COVID was coined, scientists at NIH’s intramural research program (IRP) and elsewhere began preparing for the likelihood that some people would not fully recover after infection from the novel coronavirus. Also known as post-acute sequelae of SARS-CoV-2, or PASC, long COVID is still being defined but is often described as a constellation of symptoms that persist or appear one to three months or more after an acute infection. People experiencing PASC report a markedly lower quality of life and increased rates of anxiety and depression compared with before their illness. The most common complaints include fatigue, shortness of breath, musculoskeletal pain, and a host of neurological problems such as difficulty with memory and concentration, sleep disturbances, dizziness, and changes in the senses of smell and taste.
CREDIT: NIAID
This scanning electron microscope image shows SARS-CoV-2 (round yellow particles) emerging from the surface of a cell cultured in the lab. SARS-CoV-2, also known as 2019-nCoV, is the virus that causes COVID-19.
PASC’s symptoms overlap with other post-viral syndromes that have mysterious physiological underpinnings. Ongoing complications have been documented in some survivors of two other coronaviruses—severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). And IRP researchers have investigated similar unexplained medical issues associated with Ebola outbreaks in West Africa. Unlike with MERS, SARS, and Ebola, the risk of developing PASC doesn’t seem to correlate with disease severity. Even initially asymptomatic individuals can turn up with long COVID symptoms months later.
Several NIH scientists are studying long COVID in an attempt to better understand what’s happening and how it might be treated.
Similar to chronic fatigue syndrome
At the National Institute of Neurological Disorders and Stroke (NINDS), Senior Investigator Avindra Nath, who’s known for his work on how infections affect the brain, and his colleague Brian Walitt have repurposed their observational study on myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)—yet another unexplained condition associated with a previous history of infection that looks a lot like PASC. (Results from the ME/CFS study are expected to be published in 2023.)
Nath and Walitt began their long COVID study in 2020 and have engaged the help of several institutes and centers. They aim to recruit 240 individuals.
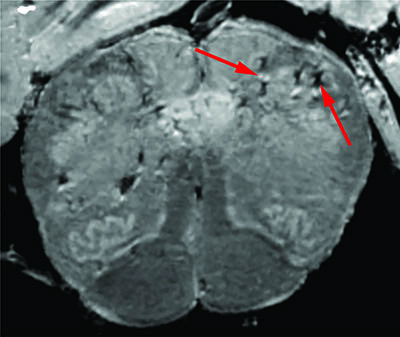
CREDIT: NINDS
A team of researchers led by Avindra Nath consistently found blood-vessel damage in the brains of COVID-19 patients but no signs of SARS-CoV-2 infections. Arrows point to light and dark spots that are indicative of blood-vessel damage observed in the study.
First, the investigators are ruling out what PASC isn’t. For example, stroke is a known complication of COVID-19. A “silent stroke” could explain cognitive difficulties reported for some people. Others who developed a chronic cough and pulmonary issues while sick might also have trouble exercising and sleeping, experiencing fatigue as a result. Viruses can also unmask or exacerbate preexisting conditions. And some patients hospitalized with severe COVID-19 will develop post-ICU syndrome, an altogether different condition in which being on a ventilator or having pneumonia can damage the lungs, heart, or kidneys.
After excluding those patients with symptoms explained by something else, Nath thinks the true prevalence of patients with lifelong PASC will likely end up being approximately 5% to 10% of the unvaccinated population. Vaccination against COVID-19 also appears to decrease the risk of developing PASC. A long-term goal of the project is to create a data-driven definition of PASC.
The protocol’s utility has also caught the attention of the U.S. Department of Veterans Affairs, which has been seeking answers for Gulf War Syndrome (GWS), another unexplained disorder with symptoms similar to ME/CFS and PASC. GWS affects approximately 30% of military veterans who served in the 1990–1991 Persian Gulf War. The NINDS team is planning to enroll patients with GWS into another trial that is similar to their PASC protocol.
Treatments for neurological symptoms
Nath’s group has found evidence of capillary damage in the brains of deceased patients who had COVID-19. He thinks that antibodies produced in response to SARS-CoV-2 infection may bind to endothelial cells in a way that damages the vessels. Blood proteins such as fibrinogen can then leak into the brain and trigger an immune reaction, causing lasting damage that could remain even if the immune system resets. NIH-funded studies have suggested similar mechanisms (Ann Neurol 91:issue 6, 2022; DOI:10.1002/ana.26350). This type of immune-mediated damage is usually associated with strokes and neuroinflammatory diseases, and the NINDS team is continuing to characterize the autopsied brain tissue to learn more (N Engl J Med 284:481–483, 2021; DOI:10.1056/NEJMc2033369).
In a separate study set to begin soon, Nath and NINDS clinical fellow Yair Mina plan to test corticosteroids and intravenous immunoglobulin (IVIg) treatments for neurological symptoms in people with PASC. Both medications have been used to treat other autoimmune and neurological disorders.
“If it’s an antibody-mediated phenomenon, the IVIg should probably work,” said Nath. But “if the antibody is causing damage to the endothelial cells, then the corticosteroid should probably work.”
Nath’s lab is also developing therapeutic compounds to target viral RNA. This type of treatment could be effective if PASC turns out to be caused by persistent viral replication. There is currently no conclusive evidence that coronaviruses persist in the body for months or years, but scientists continue to look. Tenure Track Investigator Daniel Chertow at the NIH Clinical Center has been leading a study to find SARS-CoV-2 RNA in an extensive list of body tissues, including the brain (preprint: Biol Sci, 2022; DOI.org/10.21203/rs.3.rs-1139035/v1).
No evidence of immune system dysfunction
Much of the data characterizing PASC thus far has been collected by survey or questionnaire. But since June 2020, a study led by Michael Sneller at the National Institute of Allergy and Infectious Diseases has been one of the first to conduct extensive in-person diagnostic evaluations comparing people with and without persistent symptoms.

CREDIT: NIAID
Colorized scanning electron micrograph of chronically infected and partially lysed cells (green) infected with a variant strain of SARS-CoV-2 virus particles (blue), isolated from a patient sample. Image captured at the NIAID Integrated Research Facility in Fort Detrick, Maryland.
Preliminary data on 189 post-COVID patients with (55%) and without (45%) PASC, compared with data for 120 control patients with no history of infection, showed almost no biochemical or physiological differences among the three groups that could explain PASC. There was no evidence of persistent viral infection or immune system dysfunction—two of the leading hypotheses that some scientists think could explain PASC. Increased risk for PASC, however, was noted in women and in people with a history of anxiety disorder. Sneller thinks that obesity might also turn out to be another risk factor. The study is continuing to enroll participants (Ann Intern Med 2022; DOI:10.7326/M21-4905).
“There is just a striking discoordination between the number of symptoms and the paucity of abnormal findings,” said Sneller.
Long COVID affects smell and taste
Lasker Clinical Research Scholar Paule Joseph, at the National Institute on Alcohol Abuse and Alcoholism and the National Institute of Nursing Research, is working with the NINDS-led PASC protocol team to understand how SARS-CoV-2 affects the chemical senses of taste and smell. A meta-analysis by Joseph and colleagues established taste loss as a bona fide symptom in 39% of nearly 139,000 patients with COVID-19 (Chem Senses 47:bjac001, 2022; DOI:10.1093/chemse/bjac001). Altered chemosensation often accompanies other neurological symptoms; while most patients recover in three to four months, some do not.

CREDIT: NIAAA
Paule Joseph studies the biological underpinnings that lead to variations in taste and smell perception.
Joseph plans to launch her own deep-phenotyping study of chemosensory dysfunction in patients with PASC in an attempt to understand COVID-19-induced phantosmia (detecting smells that aren’t there), parosmia (a change in the normal perception of odors such as when the smell of something familiar is distorted), and dysgeusia (a condition in which all foods taste sour, sweet, bitter, or metallic).
She anticipates that studying the SARS-CoV-2 virome and the host’s immune reaction might yield some mechanistic insights about the lingering chemosensory symptoms. A recent non-NIH study described how the virus can initiate an immune cascade that disrupts the genetic architecture and function of olfactory neurons (Cell 185:1052–1064, 2022; DOI:10.1016/j.cell.2022.01.024).
At the National Institute on Aging, Senior Investigator Josephine Egan studied cadaver tongue tissue and found that oral infection with SARS-CoV-2 occurred in taste buds. The finding suggests that the infection can disrupt stem-cell activity in taste receptors and provide a potential pathway for ongoing taste dysfunction (Am J Pathol 9:1511–1519, 2022; DOI:10.1016/j.ajpath.2021.05.010).
More questions than answers
Research into PASC continues to generate more questions than answers. Extramurally, NIH’s Researching COVID to Enhance Recovery (RECOVER) initiative is funding hundreds of clinical trials across the country to understand, prevent, and treat long-term health effects related to COVID-19. Many intramural investigators contribute to the effort by reviewing manuscripts or serving on the steering committees at RECOVER sites.
NIH intramural scientists are eligible to apply for some funding opportunities through RECOVER. Updated research opportunity announcements will be posted at https://recovercovid.org/funding.
Learn more: For an interesting talk, “Long COVID: A Brief Overview,” presented on June 2, 2022, by Ziyad Al-Aly (VA St. Louis Health Care System), go to https://videocast.nih.gov/watch=45629
This page was last updated on Thursday, June 30, 2022
