NIAMS Researchers Uncover Clues To Why Some Wounds Don’t Heal
CREDIT: ANDREW SAWAYA, NIAMS
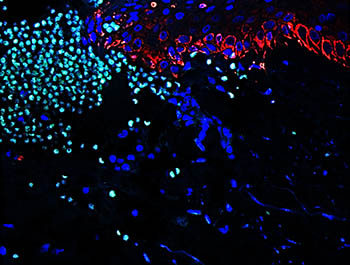
Representative image of human skin wound showing neutrophil (light green) recruitment to promote a proper inflammatory response during healing.
Unlike acute wounds, such as a paper cut or scraped knee, chronic wounds can take months to heal and leave a person at greater risk for developing infection, chronic pain, and other problems. Slow-healing foot ulcers, a complication of diabetes, are a common type of chronic wound. Diabetic foot ulcers can greatly impair a person’s quality of life and put them at risk for limb amputations or early death. Treating diabetic foot ulcers represents a significant challenge to doctors and costs billions of dollars annually in the United States.
In a new study published in Nature Communications, researchers identify defects in the wound-healing process that might explain why such wounds heal slower or not at all. The scientists also pinpoint a critical step in the pathway, the series of events contributing to wound repair, that might be a good target for developing new treatments for diabetic foot ulcers. (Nat Commun 11:4678, 2020; DOI:10.1038/s41467-020-18276-0)
The collaboration included groups led by two established skin biologists: Maria Morasso, chief of the Laboratory of Skin Biology at the National Institute of Arthritis and Musculoskeletal and Skin Diseases; and Marjana Tomic-Canic, a professor of dermatology and director of the Wound Healing and Regenerative Research Program at the University of Miami (Coral Gables, Florida). First, the scientists analyzed human tissue samples to identify the molecular culprits responsible for delayed healing. Then they confirmed their findings using specialized laboratory mice. The long-term goal of the work is to find ways to improve chronic wound healing in humans. This collaborative research was made possible by an NIH Bench-to-Bedside and Back Program Award.
Chronic Wounds Struggle to Heal. Wound healing is a normal process that involves four tightly controlled stages: hemostasis (clotting); inflammatory; proliferative (wound rebuilt with new tissue made of collagen and extracellular matrix); and maturation (wound fully closes).The second stage, the inflammatory phase, is thought to be the engine that drives the process. During this stage, white blood cells gather at the wound. These cells fight off infection and recruit other immune cells that promote tissue repair.
Chronic wounds heal very slowly because they do not advance through all the phases. Instead, chronic wounds seem to get stuck. They are unable to get past the inflammatory stage. This can lead to additional complications, such as wound infections or even limb amputations.
Scientists don’t fully understand why chronic wounds halt at this stage, so it has been difficult to design effective treatments. As evidence, current treatment options for diabetic foot ulcers are very limited, and no new therapies have been developed in the past 20 years.
Lack of FOXM1 is Key to Delayed Healing. The scientists sought to uncover what goes wrong in chronic wound healing by studying three different types of healing: injuries in the mouth (fast healing), skin injuries (average healing), and diabetic foot ulcers (slow healing). After collecting human tissue samples for each of these types of injuries, the researchers used a common gene-sequencing technique to take a close look at the specific genes and proteins—collectively known as a transcriptional network—that are involved in wound healing.
Right away, the team noticed transcriptional networks that control white-blood-cell movement and survival were revved up in mouth and skin tissues. Particularly striking was that the tissues were flooded with specialized white blood cells called neutrophils and macrophages, which are essential to wound healing.
A very different picture was revealed in diabetic foot ulcers: Transcriptional networks were weakly activated, and neutrophils and macrophages were absent from the tissue. Diabetic foot ulcers are unable to accumulate these critical white blood cells, which might partially explain why these wounds are slow to heal.
Using computer-based software to scan through transcriptional networks, the scientists pinpointed the forkhead box protein M1 (FOXM1), a regulator that triggers the recruitment of white blood cells, as a possible culprit. They found that FOXM1 was suppressed in diabetic foot ulcers but strongly activated in mouth and skin wounds.
To confirm the role of FOXM1 in a living system, the scientists turned their attention to wounded diabetic mice. One group received a chemical to block FOXM1 activity, mimicking the FOXM1-deficient environment found in diabetic foot ulcers. A second group did not. Mice treated with the FOXM1 blocker experienced reduced recruitment of immune cells and slower healing than untreated mice. These results confirmed that FOXM1 is essential for the healing of wounds, and that, without it, healing is delayed. The results suggest that finding a way to boost the activity of FOXM1 might lead to a treatment for diabetic foot ulcers and possibly other chronic wounds.
Future Directions. Morasso and Tomic-Canic agree that this study is a launching pad for multiple approaches. Going forward, the researchers aim to further their understanding of the molecular underpinnings of the disease, analyze clinical outcomes, and eventually translate their discoveries into new therapies for diabetic foot ulcers.
This article is adapted from one that appeared on the NIAMS “Spotlight on Research” website. You can read the entire article, “Researchers Uncover Clues to Why Some Wounds Don’t Heal,” posted on November 2, 2020.
This page was last updated on Thursday, March 10, 2022
