Immune Cells’ Rallying Cry Negates Cardiovascular Surgery’s Benefits
Existing Medications Could Extend Procedure’s Protective Effects
New IRP research suggests that existing medications used to treat asthma and allergies might help extend the benefits of common surgical procedures used to combat cardiovascular disease.
While modern surgery is undoubtedly a life-saving modern marvel, mucking around inside the human body rarely comes without consequences. Certain life-extending procedures meant to combat heart disease, for instance, commonly cause cardiovascular complications of their own. Fortunately, a team led by IRP researchers has identified a promising approach for staving off those surgical side effects to keep patients’ hearts robust for longer.1
When it comes to heart health, the body’s blood-carrying veins and arteries are crucial. The narrowing of the blood vessels that put the ‘vascular’ in ‘cardiovascular disease’ is a central risk factor for heart attacks because narrowed arteries can more easily get blocked, cutting off vital supplies of oxygen and nutrients that the heart needs. This, in turn, leads to heart attacks and loss of heart muscle due to oxygen deprivation.
Surgeons can extend the lives of people with heart disease by inserting tiny wire-mesh tubes known as stents into major arteries. Stents widen the vessels, allowing more blood to reach the heart and lessening the chance of a life-threatening blockage. However, much like a child forced to eat his vegetables, arteries don’t take kindly to this well-intentioned meddling. Ironically, inserting a stent into an artery triggers a process known as restenosis in which scar tissue builds up over time and causes the blood vessel to narrow once more. Without appropriate intervention, patients can end up right back where they started.
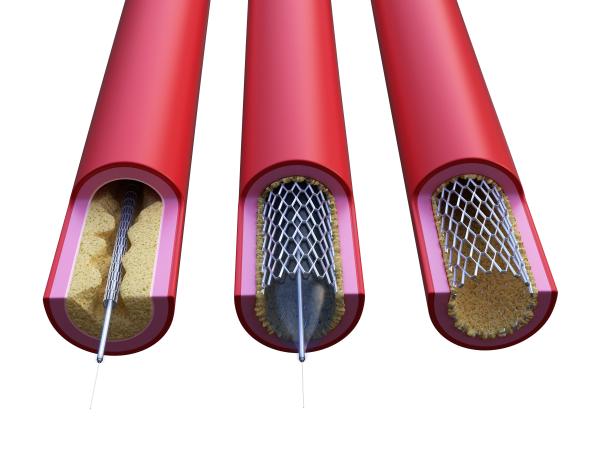
Surgeons can help prevent heart attacks by inserting stents into major arteries that have been narrowed by inflammation and the buildup of plaque.
“Restenosis is actually a wound-healing process that’s gone haywire,” says IRP senior investigator Manfred Boehm, M.D., the new study’s senior author. “It is an inadequate repair response from the blood vessel itself after it is injured. A normal blood vessel would probably do that, but diseased blood vessels do that even more. When you try to repair it with a stent, the body tries to repair what the repair did, and that’s how you get restenosis.”
“For people getting these procedures, they’re life-saving,” adds Rebecca Harper, Ph.D., the new study’s first author and a postdoctoral fellow Dr. Boehm’s lab. “They’re at the point where they’re going to have a heart attack because their heart can no longer pump the blood, so it’s a risk-benefit situation where we need to go in there and immediately address that situation and then manage the consequences further down the line.”
Current treatments for restenosis primarily address its symptoms rather than the misguided healing response at its root. In the new study, a group of scientists led by Dr. Harper and Dr. Boehm gathered compelling evidence that cells called mast cells, often thought of as the immune system’s ‘first-responders,’ are the key players responsible for restenosis. They discovered this by observing how the immune system reacted after they scratched the inside wall of mice’s arteries with a metal wire, leading to slight damage akin to what happens when a stent is inserted into an artery. Afterwards, the researchers found that mast cells in the artery’s wall released chemicals that drew other immune cells to the area, leading to inflammation and a narrowing of the artery. Genetically altered mice that lacked mast cells did not experience this restenosis-like phenomenon after injury to the artery, but when the researchers transplanted mast cells from genetically normal mice into those genetically altered mice, they began showing the same artery-narrowing immune response.
“Mast cells appear to orchestrate the immune response following injury and are therefore a logical target for therapy,” Dr. Harper says.
Fortunately, several medications already exist that quiet mast cells’ rallying cry in order to treat asthma and allergies. When the researchers gave one of those drugs — called disodium cromoglycate — to mice immediately after scratching the inside of their arteries, the animals showed much less restenosis than their untreated counterparts. Additional experiments confirmed that the drug prevented restenosis by suppressing the activity of mast cells, which in turn lowered the number of inflammatory immune cells drawn to the site of the arterial injury.

Dr. Rebecca Harper (left) and Dr. Manfred Boehm (right) led the new study.
The study’s results suggest that disodium cromoglycate or another medication with similar effects on mast cells could be used to keep blood vessels from narrowing after the insertion of a stent, thereby preserving the procedure’s benefits for longer. However, additional studies will be needed to figure out the best way to administer such a treatment, since it would likely need to start soon after the surgery and might need to be maintained for the rest of a patient’s life.
“The problem with this type of process is you don’t want to allow it to get started because it’s very difficult to reverse it,” Dr. Harper says. “It’s like trying to put toothpaste back in the tube — it’s really difficult to do that — so I would speculate it’s something you would begin immediately after surgery. There are a lot of drugs that are used in this way to try to stop the adverse effects after these types of surgeries, but they’re more symptomatic relief, whereas we’re trying to target the exact cause of this type of process.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Harper RL, Fang F, San H, Negro A, St Hilaire C, Yang D, Chen G, Yu Z, Dmitrieva NI, Lanzer J, Davaine JM, Schwartzbeck R, Walts AD, Kovacic JC, Boehm M. Mast cell activation and degranulation in acute artery injury: A target for post-operative therapy. FASEB J. 2023 Jul;37(7):e23029. doi: 10.1096/fj.202201745RR.
Related Blog Posts
This page was last updated on Thursday, September 28, 2023
