Mapping the Pathway to an Asthma Attack
IRP Research Breathes New Life into Therapies for Treatment-Resistant Asthma

IRP research focused on asthma could lead to new treatments for patients whose symptoms don’t respond to current therapies.
It may start with a wheeze, a cough, or a feeling of tightness in the chest, but the result is the same. Acute asthma attacks make sufferers feel like they’re breathing through a straw while underwater. And even between attacks, having asthma can sometimes feel like your lungs are bound in tight bandages that make it difficult to take a deep breath.
World Asthma Day, observed this year on May 2, raises awareness of asthma, a common inflammatory disease that causes difficulty breathing in more than 260 million people worldwide, including 25 million in the U.S., roughly 8 percent of the country’s population. While many people can control their symptoms by taking medications and limiting certain activities, the condition still causes significant illness and even death.
“While the medical community has done an outstanding job advancing the understanding and treatment of asthma, there are still about 3 to 5 percent of patients with severe disease who suffer most of the bad consequences due to asthma,” says IRP senior investigator Stewart Levine, M.D. “We need to develop better treatments to offer options to patients who don't respond to what's currently available.”
Dr. Levine’s research aims to shed light on the biological processes that influence asthma symptoms in the hopes that better understanding them can lead to more effective treatments, especially for those who have a type of asthma that doesn’t respond well to existing treatments. Those treatments currently include inhaled corticosteroids designed to immediately reduce airway swelling and long-acting medications that widen patients’ breathing passages.
In addition to the airway constriction and mucus buildup that are characteristic of asthma, the disease also causes significant inflammation in the tissue that lines the lungs' airways. That inflammation can further be divided into two categories. The first, called type 2-high, is characterized by excess immune cells called eosinophils. Encounters with an allergen, such as dust mites, pollen, or pet dander, can trigger production of eosinophils, which then induce swelling in the lungs’ airways.
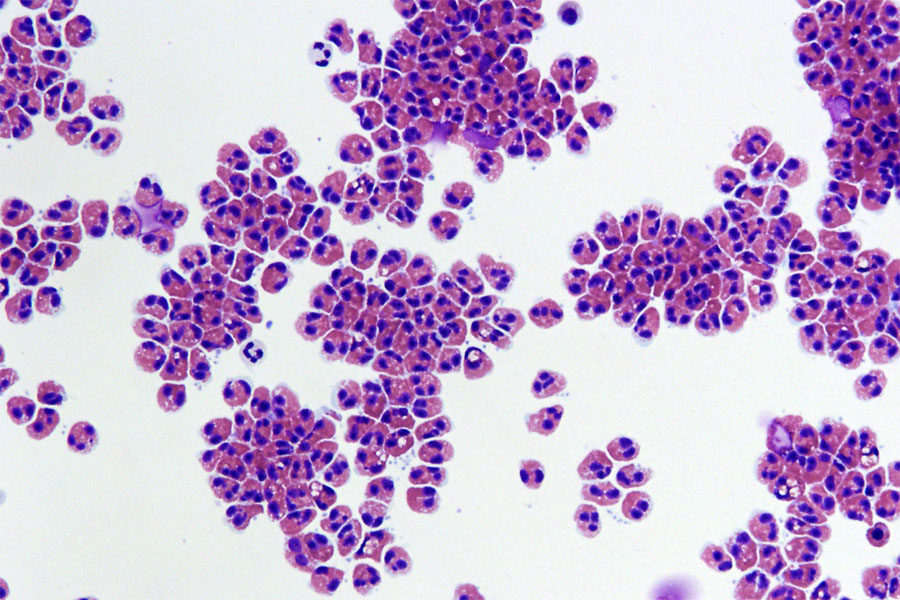
Human eosinophils, a type of white blood cell, are shown isolated from blood. These cells store and release packages of inflammatory proteins (red) that can damage tissues. Image courtesy of Julie Caldwell and Cincinnati Children’s Hospital Medical Center
“Thanks to the knowledge generated by the asthma and immunology research communities, the specific molecules that drive eosinophilic inflammation have been identified, and now we can treat patients with laboratory-made inhibitors called monoclonal antibodies, which can counteract the eosinophilic inflammation if patients don’t respond to standard treatment,” Dr. Levine says.
However, not everyone has eosinophilic asthma. Patients who are said to have ‘type 2-low asthma,’ tend to have elevated levels of a different type of inflammatory immune cell called neutrophils. Sometimes, though, their levels of immune cells appear perfectly normal. In fact, Dr. Levine says type 2-low asthma is diagnosed by the absence of markers for type 2-high asthma.
“The ongoing unmet need is to develop a better understanding of, and a better treatment for, patients with type 2-low disease,” he says.
His search for a solution for type 2-low disease began with an effort to understand how genes behave in laboratory mice that were exposed to dust mites to mimic an allergic asthma response. Dr. Levine’s team specifically looked for genes that were activated upon allergen exposure but then didn’t return to normal following treatment with corticosteroids.
“One of the genes we found was apolipoprotein E, and that was our clue that we would want to start there,” Dr. Levine says. “At the time, there was nothing known about the role of ApoE in asthma, so we said, ‘Okay, let’s study that.’ And it worked so well that it has taken over our research laboratory program for the last 15 years.”
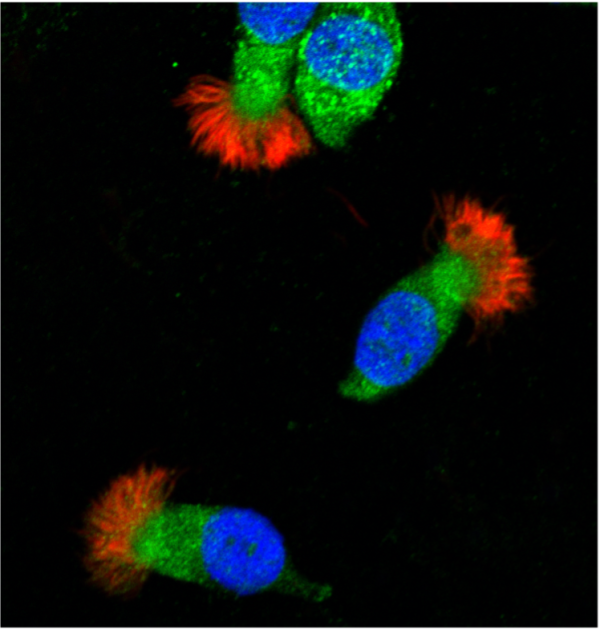
These human airway epithelial cells have low-density lipoprotein (LDL) receptors (shown in green), which bind apolipoprotein E. The cell nucleus is shown in blue. Image courtesy of Elizabeth Gordon, Ph.D.
His IRP lab now focuses on two specific and apparently opposing apolipoprotein pathways. The first is controlled by apolipoprotein E (ApoE), an important protein in particles that transport fat molecules called lipids, including cholesterol, through the bloodstream and into cells. These cholesterol carriers include the low-density lipoproteins (LDL) and very low-density lipoproteins (VLDL) we know as ‘bad’ cholesterol. In addition to its role in cholesterol buildup and heart disease, ApoE has also been associated with Alzheimer’s disease and susceptibility to infectious diseases. A study led by a staff scientist in Dr. Levine’s lab, Xianglan Yao, M.D., Ph.D., showed that during allergic asthma caused by dust mites, ApoE in the lungs binds to receptors for LDL particles on the cells lining the airways, leading to a decrease in airway constriction and mucus production. 1
The second pathway Dr. Levine studies involves apolipoprotein A1 (ApoA1), an important component of high-density lipoproteins (HDL), the ‘good’ molecule that carries cholesterol out of cells. As they do this, HDL particles cause an anti-inflammatory effect within the body. In collaboration with researchers in the lab of IRP senior investigator and lipoprotein expert Alan Remaley, M.D., Ph.D., Dr. Yao and other members of Dr. Levine’s team exposed mice bred to lack ApoA1 to an allergen from egg whites called ovalbumin. The allergen increased the animals’ levels of neutrophils, the type of immune cell sometimes seen in elevated numbers in type 2-low asthma. Moreover, the mice without ApoA1 had a greater elevation of neutrophils in response to the allergen than regular mice that still had ApoA1, suggesting that ApoA1 was protective against airway inflammation caused by neutrophils in type 2-low asthma.
“That was relevant because while we now have a lot of treatment options for type 2-high, severe asthmatics who don’t respond to steroids, there are very few options for people with type 2-low asthma,” says Dr. Levine.
Dr. Stewart Levine
One new treatment option might come from a man-made peptide developed in Dr. Remaley’s lab that can mimic the ability of ApoA1 to remove cholesterol from cells. While this is obviously helpful in treating heart disease, it also turned out to be useful in suppressing inflammatory cells in asthma. In fact, Dr. Levine’s and Dr. Remaley’s labs found that the new molecule, called ApoA1 mimetic peptide, helped reduce airway inflammation in mice caused by either eosinophils or neutrophils.
“These findings suggest that if you could generate an inhaled version of an ApoA1 mimetic peptide, that might be a novel treatment approach that could be used for both type 2-high and type 2-low asthma,” says Dr. Levine.
Building on that finding, a study from Dr. Levine’s lab, led by IRP staff clinician Amisha V. Barochia, M.B.B.S., M.H.S., showed that asthma patients who had more large HDL particles — the kind in which ApoA1 is found— in their blood had better lung function.2 In addition, Dr. Barochia and Dr. Levine have found that patients with type 2-high asthma who have higher HDL levels tend to have lower amounts of the eosinophil cells that contribute to their symptoms.3
ApoA1 is not the only intriguing therapeutic target Dr. Levine has helped identify. He has also collaborated with IRP senior investigator Jay Chung, M.D., Ph.D., an expert in DNA-dependent protein kinase (DNA-PK), an enzyme present in certain immune cells in the lungs. Together, they found that DNA-PK is activated by exposure to allergens like dust mites, so inhibiting it may prove to be helpful for asthma patients.4
Learn about Dr. Levine's and Dr. Chung's research collaboration in this video.
Despite these promising discoveries, it remains a challenge to translate the findings into treatments for both types of asthma. Dr. Levine hopes to find a pharmaceutical company interested in tackling that next step while he continues his research.
“Our long-term goal is to define new pathways and suggest treatments,” he says. “We do the science with the goal that our discoveries will get picked up and translated into novel treatments that can potentially improve the lives of patients who suffer from asthma.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Yao X, Fredriksson K, Yu ZX, Xu X, Raghavachari N, Keeran KJ, Zywicke GJ, Kwak M, Amar MJ, Remaley AT, Levine SJ. Apolipoprotein E negatively regulates house dust mite-induced asthma via a low-density lipoprotein receptor-mediated pathway. Am J Respir Crit Care Med. 2010;182(10):1228-38. doi: 10.1164/rccm.201002-0308OC.
[2] Barochia AV, Kaler M, Cuento RA, Gordon EM, Weir NA, Sampson M, Fontana JR, MacDonald S, Moss J, Manganiello V, Remaley AT, Levine SJ. Serum apolipoprotein A-I and large high-density lipoprotein particles are positively correlated with FEV1 in atopic asthma. Am J Respir Crit Care Med. 2015;191(9):990-1000. doi: 10.1164/rccm.201411-1990OC.
[3] Amisha V. Barochia, Elizabeth M. Gordon, Maryann Kaler, Rosemarie A. Cuento, Patricia Theard, Debbie M. Figueroa, Xianglan Yao, Nargues A. Weir, Maureen L. Sampson, Mario Stylianou, David F. Choy, Cecile T.J. Holweg, Alan T. Remaley, Stewart J. Levine. High density lipoproteins and type 2 inflammatory biomarkers are negatively correlated in atopic asthmatics. J Lipid Res. 2017;58(8): 1713-1721. doi.org/10.1194/jlr.P077776.
[4] Mishra A, Brown AL, Yao X, Yang S, Park SJ, Liu C, Dagur PK, McCoy JP, Keeran KJ, Nugent GZ, Jeffries KR, Qu X, Yu ZX, Levine SJ, Chung JH. Dendritic cells induce Th2-mediated airway inflammatory responses to house dust mite via DNA-dependent protein kinase. Nat Commun. 2015 Feb 18;6:6224. doi: 10.1038/ncomms7224.
Related Blog Posts
This page was last updated on Friday, June 16, 2023
