Synthetic Materials Influence Body’s Healing Response
Research Could Lead to New Strategies for Treating Injuries
A new IRP study detailed how certain surgically implanted materials influence the body’s healing process.
Modern medical advances mean that many people are not “only flesh and blood.” Mechanical devices and substances created in medical labs are commonly replacing or being added to parts of people’s bodies. A new IRP study has shed light on how some of those materials might influence the body’s healing process, providing insights that could eventually spur the creation of new ones that influence the behavior of the body’s immune system and allow doctors to better direct how the body repairs itself.1
Our bodies depend on the immune system to fix damage, but immune cells can get overzealous, leading to inflammation, scarring, and slower healing. This can be particularly problematic when the immune system attacks health-promoting devices or materials surgeons have introduced into the body. Given the right conditions, though, immune cells can be coaxed to perform better and speedier repairs and cause less destruction. Researchers like IRP Stadtman Investigator Kaitlyn Sadtler, Ph.D., seek to discover what those conditions are.
“The way the immune system is behaving can affect different stages of healing,” Dr. Sadtler explains. “When the immune system comes up against a bacterial infection, it’s operating in a specific environment. It’s like when we go outside and it’s hot, so we wear a T-shirt. On the other hand, the immune system might see a parasite instead, and it will act differently because it’s in a different environment. Now it’s winter, so you’re going to put a sweater on.”
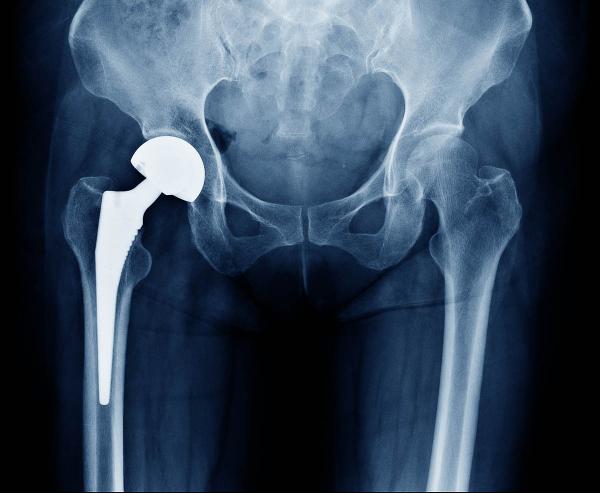
Surgeons commonly introduce synthetic substances into the body, such as in knee and hip replacements, which are often made out of a form of plastic that can cause inflammation and scarring over time due to its influence on the immune system.
Doctors can adjust the immune system’s thermostat by utilizing different materials in medical procedures. For instance, a form of plastic called polyethylene is commonly used for knee and hip replacements because it is durable and can be easily molded into different shapes, but according to Dr. Sadtler, “when it gets old and wears, you end up getting inflammation around the debris, tissue gets destroyed, and scar tissue forms.” Another frequent player in medical procedures, called decellularized extracellular matrix (ECM), helps wounds heal and is employed to fix injuries in which a section of tissue has been damaged, such as breast reconstruction, repairing traumatic injuries, and treating diabetic foot ulcers.
“Knowing about how different materials change the immune response might allow us to modulate what’s good and what’s bad for healing in order to make better materials and devices,” Dr. Sadtler says. “And it’s also useful for clinicians’ own knowledge. If you know what’s going to happen when you put a certain material into a wound, that’s very helpful.”
“You want to make sure you have enough immune activation so that there’s healing, but you also don’t want to destroy yourself in the process or lay down a horrible scar,” Dr. Sadtler adds. “It’s a really balanced system.”
In the new study, Dr. Sadtler’s team implanted either polyethylene powder or decellularized ECM into the gap left when a section was surgically removed from the muscles of mice. Other mice were left untreated other than with an injection of salt water into the wound area. The IRP scientists then examined how the wounds healed and various types of immune cells behaved in the different groups of mice.
The researchers found that the injury site contained more of two proteins, E-cadherin and interleukin-4, when it was treated with the pro-healing ECM material. E-cadherin is a molecular anchor that keeps cells attached to one another, while IL-4 promotes muscular repair by helping individual muscle cells link together into muscle fibers.
In addition, the scientists discovered that certain types of immune cells were recruited to the site of the injury in higher numbers when it was treated with the pro-healing ECM material. For example, ECM brought in more of a variety of immune cell that calms the immune system down. The ECM-treated wounds also contained many more immune cells called eosinophils, while wounds treated with the polyethylene powder that tends to produce scarring and inflammation contained more immune cells called neutrophils.
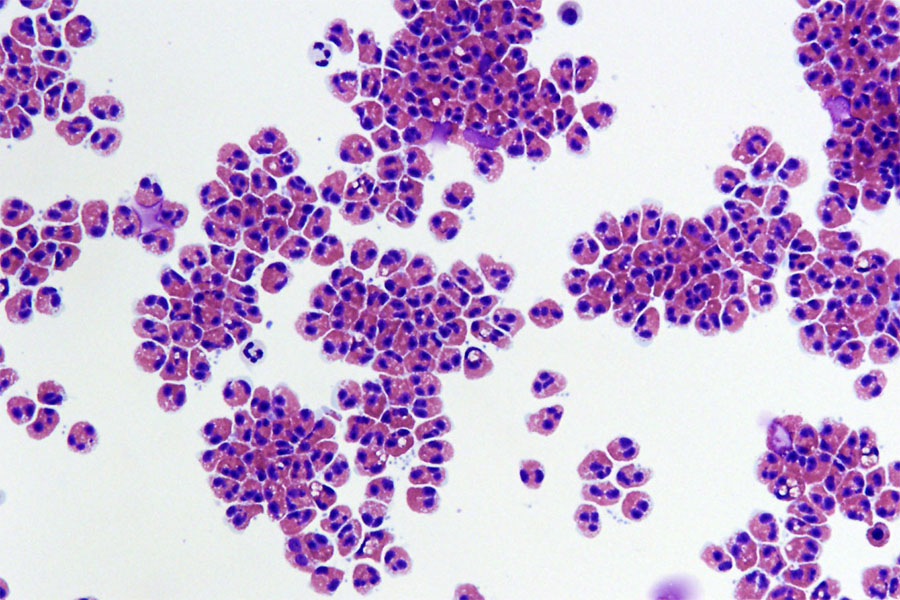
Human eosinophils isolated from blood. Image credit: Cincinnati Children’s Hospital Medical Center/Julie Caldwell
“Eosinophils are more associated with asthma and wound healing, whereas the neutrophils are more associated with those initial inflammatory responses to things like bacterial infections,” Dr. Sadtler explains. “Of course, other types of neutrophils exist, but the canonical role for neutrophils is to come in, fire those cannons, and murder the bacteria.”
The ECM-treated wounds also contained more immune cells called ‘natural killer’ cells — specifically, a variety of natural killer cell that secretes a substance called XCL1. This increased concentration of XCL1 served as an enticement for yet another type of immune cell called cDC1 dendritic cells. That variety of dendritic cell has been previously seen in the immune response to cancer, an intriguing link given that tumors have been called “the wound that never heals,” Dr. Sadtler says.
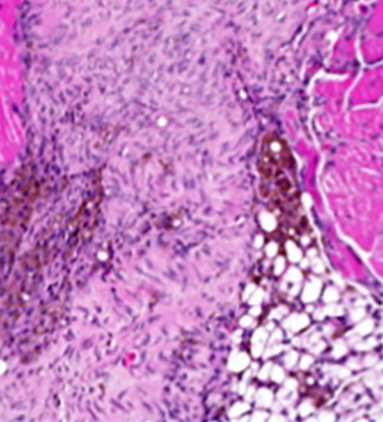
Healing was disrupted in mice lacking cDC1 dendritic cells, resulting in abnormalities such as fat deposits in the muscle where they should not have been (bottom-right).
Experiments in mice that lacked those cDC1 dendritic cells reinforced the idea that they promote healing. For example, those mice had deposits of fats in their damaged muscles where fat should not be, and their muscles underwent greater amounts of fibrosis, a process that leads to scarring. In addition, the researchers saw signs in those mice that undamaged muscle fibers near the injury site were dying and undergoing a process called calcification that turned them into bone-like tissue.
The sorts of observations Dr. Sadtler’s team made in the study are the first step on the path towards medical interventions that spur the immune system to act in specific ways; in fact, her research group is currently leveraging them to design new materials with that goal in mind. Such strategies could help patients heal faster after injuries or surgery or prevent the immune system from wearing down surgically implanted devices like hip replacements over time, making them last longer. Even further down the line, Dr. Sadtler says she has a “pie-in-the-sky goal” to use her lab’s discoveries to create vaccines that will enhance people’s ability to recover from injury.

Dr. Kaitlyn Sadtler
“Once you understand the biology and you’ve got a target, it makes it a lot easier to make a therapeutic,” she says. “As we go on, we’re going to continue learning about ways our immune system is responding to injuries and responding to materials, so we’re going to keep digging at that basic biology, but at the same time we’re going to start to build materials that manipulate these pathways to hopefully promote tissue regeneration.”
“It’s an exciting intersection of basic immunology and materials bioengineering that we’re thrilled to work in,” Dr. Sadtler continues. “It’s got some awesome folks in the field, and I think that we’re going to see a lot of advancements by pushing forward this envelope that’s kind of in between that understanding of immunology and tissue regeneration. It’s fun to work on that. Blurring the lines between science fiction and science fact is the best.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Lokwani R, Josyula A, Ngo TB, DeStefano S, Fertil D, Faust M, Adusei KM, Bhuiyan M, Lin A, Karkanitsa M, Maclean E, Fathi P, Su Y, Liu J, Vishwasrao HD, Sadtler K. Pro-regenerative biomaterials recruit immunoregulatory dendritic cells after traumatic injury. Nat Mater. 2023 Oct 23. doi: 10.1038/s41563-023-01689-9.
Related Blog Posts
This page was last updated on Tuesday, January 9, 2024
