High-Fat Diet Alters Brain’s Response to Food
Mouse Study Identifies Neurological Obstacle to Dietary Improvements

A new IRP study suggests that a diet heavy on high-fat fare can cause neurological changes that make healthier food less satisfying.
Every morning, thousands of Americans wake up intending to eat more healthfully, only to find themselves chowing down on a greasy burger at dinnertime. In addition to the many biological and socioeconomic obstacles to healthy eating, a salad can just plain seem unappealing compared to a plate of crispy fries. According to new IRP research, a high-fat diet can dramatically alter how the brain responds to food in ways that make a more wholesome meal less enticing and satisfying.1
More than 40 percent of American adults are obese, in addition to nearly a fifth of children and teens. A large contributor to the obesity epidemic is the consumption of large amounts of energy-dense, ‘hyper-palatable’ foods that are often high in fat, such as fast food and desserts. Eating less of this sort of food and increasing intake of more nutritious alternatives can lead to both weight loss and improved health, but maintaining a healthy diet is easier said than done.
“I think we’ve all had that experience of deciding ‘I’m going to start eating healthy,’ but sticking to that is difficult,” says IRP senior investigator Michael Krashes, Ph.D. “There has to be some way that the brain is responsible for this because it’s not like this is affecting only a small portion of the human population. This is a phenomenon that happens in almost everyone.”
Past studies by Dr. Krashes and other neuroscientists have found that a set of neurons that release a molecule called agouti-related peptide (AgRP) play a major role in food consumption.2 Located in a brain region called the hypothalamus, these cells are highly active when we are hungry, and this activity is thought to produce the negative feelings associated with hunger.3 Consuming food calms these neurons, thereby curbing the desire to eat.4
In a new study, Dr. Krashes’ team — led by IRP postdoctoral fellow Christopher Mazzone, Ph.D., and IRP graduate student Jing Liang-Guallpa — investigated whether a high-fat diet might change the way these AgRP neurons function. The researchers provided mice either with only a standard laboratory mouse diet or with both that typical rodent chow and food that was much higher in fat. Unsurprisingly, the mice with access to high-fat fare all but completely ignored the standard food and gained substantial amounts of weight. However, when those animals lost access to the high-fat chow, they continued to shun the standard food, consuming so little of it that they lost weight. Their aversion to the standard chow remained even when they were forced to fast overnight to boost their appetites, though they happily consumed large amounts of high-fat food after fasting if provided with it. These behavioral changes persisted after two weeks without access to the high-fat chow and did not differ significantly in mice that gained different amounts of weight on the high-fat diet.
“Even though the mice are in need of calories and are really hungry, they’ll basically go on a hunger strike and refuse to eat the standard diet for a long period of time,” Dr. Krashes says. “After we’ve given them this high-fat diet, they like it so much that they now don’t want to even touch that other type of food.”
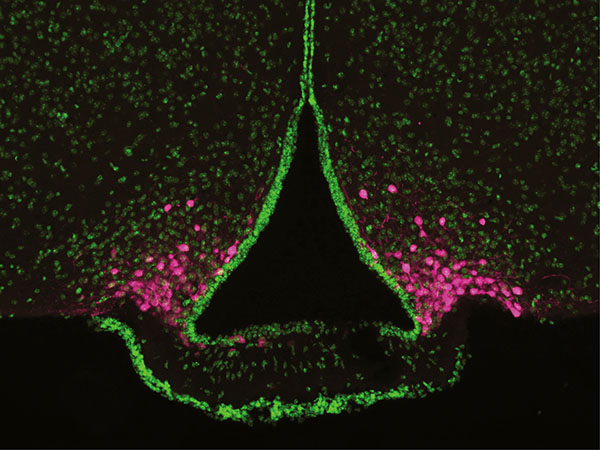
AgRP neurons (magenta), located at the base of the brain’s hypothalamus, play a critical role in hunger.
The IRP team also recorded the electrical activity of AgRP neurons in fasted animals. These experiments showed that consumption of standard rodent chow suppressed AgRP neuron activity much less in hungry mice that had previously received high-fat food compared to mice that had not, suggesting that the standard chow did little to satisfy the appetites of the mice that had tasted more delectable fare. Once again, this remained the case two weeks after the animals provided high-fat food were switched back to the standard diet. High-fat food, on the other hand, never failed to tamp down AgRP neuron activity.
Animals exposed to the high-fat diet shunned the standard chow even when Dr. Krashes’ team boosted their drive to eat by directly stimulating their AgRP neurons. However, this direct stimulation successfully increased consumption of the standard chow in animals that had never been on a high-fat diet.
Collectively, these intriguing findings, along with changes in several other brain areas that the IRP scientists observed in mice given high-fat food, point to a neurological explanation for why it is so difficult to make lasting improvements to one’s diet. Dr. Krashes’ team plans to follow up its new study by investigating how a high-fat diet changes the behavior of genes in different populations of brain cells in mice. This sort of research could one day lead to interventions that help people make long-term changes to their eating habits.
“All hope is not lost,” Dr. Krashes says. “The good thing about identifying the circuitry and the molecules that play a role in these feelings of satiety and hunger is that pharmaceutical companies now have specific therapeutic targets to try to direct their drugs toward. The more we discover, the more we make progress on this.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] High-fat food biases hypothalamic and mesolimbic expression of consummatory drives. Mazzone CM, Liang-Guallpa J, Li C, Wolcott NS, Boone MH, Southern M, Kobzar NP, de Araujo Salgado I, Reddy DM, Sun F, Zhang Y, Li Y, Cui G, Krashes MJ. Nat Neurosci. 2020 Aug 3. doi: 10.1038/s41593-020-0684-9.
[2] Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. J Clin Invest. 2011 Apr;121(4):1424-1428. doi: 10.1172/JCI46229.
[3] Neurons for hunger and thirst transmit a negative-valence teaching signal. Betley JN, Xu S, Cao ZFH, Gong R, Magnus CJ, Yu Y, Sternson SM. Nature. 2015 May 14;521(7551):180-185. doi: doi: 10.1038/nature14416.
[4] Sensory detection of food rapidly modulates arcuate feeding circuits. Chen Y, Lin Y, Kuo T, Knight ZA. Cell. 2015 Feb 26;160(5):829-841. doi: 10.1016/j.cell.2015.01.033.
Related Blog Posts
This page was last updated on Tuesday, May 23, 2023
