Teaching an Old Vaccine New Tricks to Thwart Tuberculosis
The IRP’s Mario Roederer and Robert Seder Discuss the Science Behind the Headlines
These Mycobacterium tuberculosis bacteria are the cause of tuberculosis (TB). A pair of IRP researchers and their collaborators recently discovered that administering the century-old TB vaccine in a new way could dramatically improve protection from the illness.
Some say that if something’s not broken, then don’t fix it, but that doesn’t mean there’s no room for improvement. At least, those were the thoughts of IRP senior investigators Mario Roederer, Ph.D., and Robert Alan Seder, M.D., who recently found that the century-old tuberculosis (TB) vaccine is far more effective when administered via injection into a vein (IV) rather than into the skin, which has long been the standard way it is given. This major breakthrough received extensive media coverage, including a story in the New York Times. We went Behind the Headlines to get the inside scoop on this potentially life-saving discovery.
TB is a severe, contagious bacterial disease that primarily affects the lungs but can also infect other organs. The disease can be fatal without treatment, particularly in those with weakened immune systems, such as patients with HIV. Because of its serious health consequences, people around the world have received the TB vaccine at birth intradermally — meaning via an injection into the skin — since its initial development in 1921. While this is effective at hindering the spread of TB throughout the entire body, it has limited efficacy at stopping the TB bacterium from colonizing the lungs.
Unfortunately, despite widespread vaccination, TB was responsible for 1.5 million deaths and 10 million illnesses worldwide in 2018. Because of this, Drs. Roederer and Seder decided to challenge the status quo by evaluating different doses and routes for administering the vaccine.
“The TB vaccine that has been used for 100 years had not been optimized and studied in the same way that vaccines in development today are,” says Dr. Roederer. “In the old days, researchers decided to administer the vaccine intradermally, and there wasn’t much research done on alternative administration routes."
More than a decade ago, Dr. Roederer was studying the simian immunodeficiency virus (SIV), a virus closely related to HIV, in non-human primates. Specifically, his group was investigating various doses and administration routes for SIV vaccines, including vaccinating directly at the sites of infection or via an aerosol spray inhaled directly into the lungs. Through those experiments, his team found that the latter method of SIV vaccine administration triggered a much stronger response by immune cells called T cells in the lungs than when the vaccine was delivered by injection into the muscle, which is a standard route of immunization. This increase in T cells in the lungs was important because T cells are responsible for protecting the body against foreign invaders like viruses and bacteria, including those responsible for tuberculosis.
These findings encouraged Drs. Roederer and Seder to explore alternative administration methods for the TB vaccine. Specifically, because administering the SIV vaccine via an aerosol spray increased accumulation of T cells in the lungs, the researchers hypothesized that aerosol administration, rather than the long-standing intradermal delivery method, might be the best way to administer the TB vaccine, since the bacterium that causes TB enters the body via the lungs.
T cells like the one pictured here are crucial to protecting the body from viruses like HIV and bacteria like those that cause tuberculosis.
“When we saw this robust T cell response in the lungs, a lightbulb went off,” Dr. Roederer explains. “In order to control infection and prevent disease onset, a TB vaccine must evoke strong responses from the immune system’s T cells, specifically those in the lungs.”
To test their hypothesis, the two senior IRP researchers partnered with JoAnne Flynn Ph.D., of the University of Pittsburgh to assess whether administering a TB vaccine via aerosol spray of a genetically engineered viral agent would increase T cells in the lungs and improve protection from TB in non-human primates. Surprisingly, while there were robust T cell responses in the lungs following the vaccination, there was limited protection.
Not to be discouraged, the investigators continued to test whether alternative immunization routes could both improve protection from TB and increase the gathering of T cells in the lungs. To further their studies, the reseachers made two major changes. First, they switched from their genetically-engineered vaccine to the standard TB vaccine that prevents the tuberculosis bacterium from spreading throughout the body but does poorly at preventing TB infection of the lungs, which is the main cause of death among TB patients. The second major change was administering the vaccine intravenously, an idea that stemmed from Dr. Seder’s previous research showing that IV administration of a malaria vaccine significantly enhanced protection from malaria compared to an intradermal vaccination.
“The data from my earlier malaria research provided the conceptual foundation for using intravenous administration to stimulate the kind of T cell responses in the lung that would be necessary to provide protection,” explains Dr. Seder. “Indeed, administering the TB vaccine intravenously induced higher T cell responses in the lungs compared to administering it via aerosol or intradermally, as had traditionally been done. Most importantly, this provided complete protection from tuberculosis infection, a truly significant breakthrough.”
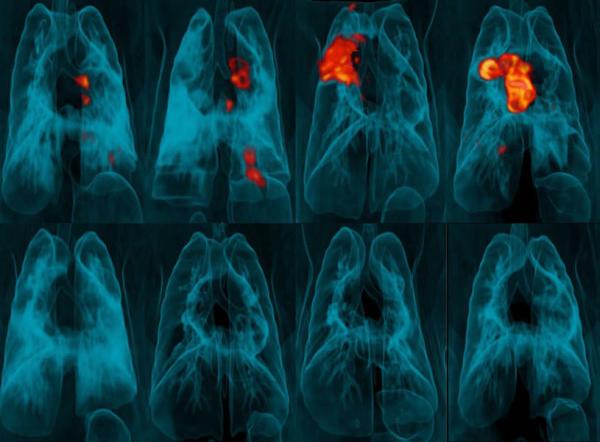
These PET scans of monkeys’ lungs show tuberculosis-induced inflammation in red and yellow. The animals in the top row received the TB vaccine via the traditional, intradermal route, whereas those in the bottom row received the vaccine via injection into a vein.
A major discovery such as this one doesn’t happen alone. Drs. Roederer and Seder utilized the knowledge and expertise of several colleagues in collaborations that were essential to the study. Specifically, IRP staff scientist Patricia Darrah, Ph.D., played a vital role in the design and execution of the study. In addition, the team relied on Dr. Flynn to monitor the TB infection after administering the vaccine using a PET-CT scanning technology that her lab had created. On top of that, they were able to collect detailed measurements of a large number of individual T cells using a technology that had been in development in Dr. Roederer’s laboratory for 20 years. Finally, the team collaborated with Alex Shalek, Ph.D., of the Massachusetts Institute of Technology to identify how the activity of genes in lung cells was related to protection from TB.
“It was a combination of about four to five key technologies that allowed us to come up with the incredible dataset that was published,” Dr. Roederer says. “Additionally, I truly believe that our findings would not have been possible without the impeccable cross-functional collaboration with a number of brilliant researchers.”
Building on their findings and collaborations, Drs. Roederer and Seder and their colleagues at the NIH’s Vaccine Research Center have taken steps to move this new vaccination approach into clinical development for study in humans. While vaccine innovations like this have potentially life-saving implications, the researchers are moving slowly and strategically to ensure they adequately address safety concerns and other hurdles.
“There have been millions of people around the world infected by TB, millions die every year, and it’s the number one cause of death in HIV-infected people,” explains Dr. Roederer. “Because of this, it’s extremely important to improve prevention of this disease.”
Visit our news page to learn more about this research, and subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
Related Blog Posts
This page was last updated on Wednesday, May 24, 2023
