Mouth Microbes Turn Treasonous in Gum Disease
Four Questions with Dr. Niki Moutsopoulos
An abnormal and unhealthy population of bacteria in our mouths triggers specific immune cells to cause inflammation and attack the gums and bones that support our teeth, according to IRP research.
Our mouths are teeming with bacteria, a microbial ecosystem known as the oral microbiome. While these microbes are typically benign, under certain circumstances they can turn harmful and contribute to oral diseases such as periodontitis, a form of chronic gum disease characterized by microbe-driven inflammation of the soft tissues and bone that support our teeth. According to the Centers for Disease Control and Prevention (CDC), roughly 65 million Americans aged 30 or older have some degree of periodontitis. In its early stage, known as gingivitis, the gums become swollen and red due to inflammation, which is the body’s natural response to the presence of bacteria. If the condition worsens, it can lead to loose teeth and, eventually, bone or tooth loss.
NIH senior investigator Niki Moutsopoulos, Ph.D., head of the Oral Immunity and Inflammation Section at the National Institute of Dental and Craniofacial Research (NIDCR), studies periodontitis and aims to understand the immune system’s role in driving this destruction. In a 2018 study, she and her team of IRP researchers and outside collaborators discovered that an abnormal and unhealthy population of microbes in the mouth causes specialized immune cells, known as T helper 17 (Th17) cells, to trigger inflammation and destroy tissue, leading to periodontitis.
In recognition of World Oral Health Day, I sat down with Dr. Moutsopoulos to discuss her research on periodontitis, how these sorts of scientific collaborations lead to breakthroughs, and the next phase of her experiments.
What was the importance of conducting this research?
“Periodontitis is a very common disease that affects a large proportion of the general population. Consequently, understanding disease mechanisms and identifying therapeutic targets is a very important and widely pursued topic of investigation. Beyond understanding the disease itself, studying periodontitis also provides an opportunity to improve our understanding of how our bodies interact with our microbiomes, a topic of interest to a broad audience of biomedical scientists.
“My lab and our collaborators specifically want to understand how the local immune response in the oral cavity changes in the setting of periodontitis and can affect disease. For years, we’ve known that bacteria can cause inflammation in the gums, but while brushing, flossing, and dental cleanings help control inflammation, they do not eliminate it, suggesting the existence of other contributing factors.
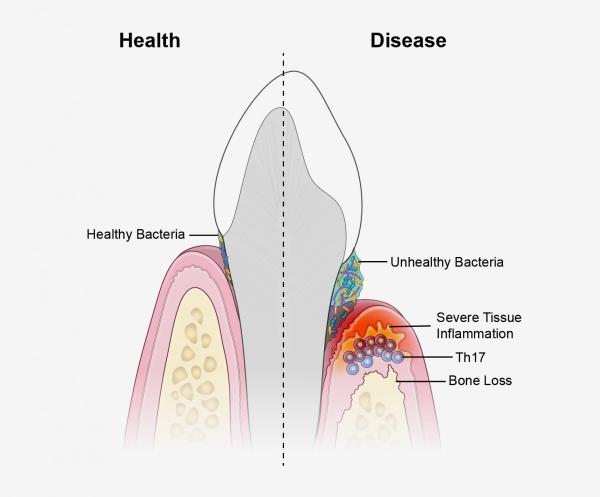
This diagram compares the conditions in a healthy mouth (left) to those in a mouth suffering from periodontitis (right).
“Previous studies had found that immune cells called Th17 cells, which secrete an inflammation-inducing chemical called IL-17, become more abundant in the gums of individuals with periodontitis. This finding piqued our interest because these cells normally live in so-called barrier sites that keep harmful substances from getting into the body, such as the mouth, skin, and digestive tract. They are also known to provide protection at these entry sites where germs make first contact with the body. However, in many illnesses, including autoimmune and chronic inflammatory disorders like psoriasis and colitis, these helper T cells have been linked to disease. As a result, we really wanted to know how these cells become dysregulated in periodontal disease and understand their role in triggering tissue destruction and bone loss. Ultimately, in our 2018 study, we discovered that unhealthy changes to the oral microbiome trigger an increase in the number of Th17 cells in the gums, thereby establishing a link between oral bacteria and the inflammation we see in periodontitis.”
What was the most challenging aspect of completing this research? Were there any significant hurdles?
“Scientific research always poses challenges — that’s the beauty of it. Every study has its issues, and ours are no different. We experienced the general difficulties that most scientists do, such as needing to develop techniques to answer our questions and completing the same experiments multiple times to be confident about our results. For example, we had to create specialized approaches that would allow us to study the immune responses within oral tissues that we were specifically interested in. In the years before our 2018 study, we invested in a large effort to dissect oral tissues from both humans and mice and isolate immune cells from these tissues in order to characterize and study them. We also performed a large study in the preceding years examining immune cell populations in the mouth’s mucous membranes in healthy individuals. These previous studies created a baseline for us to begin scrutinizing shifts in inflammatory responses linked to disease.”
What collaborations were crucial to this research?

Dr. Niki Moutsopoulos
“We've had a lot of fruitful collaborations that have helped us along the way. The truth is that we really could not do most of our work without the fantastic partnerships we have formed both within the IRP and with scientists outside the NIH. All our work is highly collaborative.
“For several years, my lab has been studying a rare form of periodontal disease called leukocyte adhesion deficiency 1, or LAD1, in collaboration with the group of Dr. Steve Holland in the National Institute of Allergy and Infectious Diseases. It was really in the setting of this rare disease that we first encountered immune cells over-producing the inflammatory molecules IL-17 and IL-23 in oral tissues suffering from periodontal destruction. These findings in the rare disease setting stimulated us to also investigate relevant responses in the setting of a common disease like periodontitis. In addition, for many years we have worked very closely with the research group of the University of Pennsylvania’s Dr. George Hajishengallis both in our studies of LAD1 and common forms of periodontitis. It was in fact George’s group that helped us set up animal models of periodontitis in our laboratory.
“These critical partnerships have not only allowed us to expand our studies with new experimental techniques but also provide a continuous platform for intellectual exchange and stimulation.”
How have you expanded on this topic and your research? What are your plans for the future?
“From a basic science standpoint, we continue to dissect mechanisms involved in the way oral microorganisms activate Th17 cells and the mechanisms by which these cells cause tissue destruction. From a clinical and translational standpoint, we are excited that some of our work has already made its way into the clinical setting. In the case of LAD1-associated periodontitis, in collaboration with Dr. Holland’s group, we have already treated one patient with a targeted therapy that inhibits immune cells’ release of the inflammatory IL-17 molecule by blocking the action of a related molecule, IL-23. We had very encouraging clinical results in this one case and have now initiated a clinical trial to treat patients with LAD1 disease at the NIH Clinical Center using this therapy.”
Visit our Accomplishments page for more information on Dr. Moutsopoulos' research, and subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
Related Blog Posts
This page was last updated on Tuesday, January 30, 2024
