The Perplexing Pancreas
Multidisciplinary Research Tackles an Increasingly Common Pancreatic Disorder
BY MICHAEL TABASKO, THE NIH CATALYST
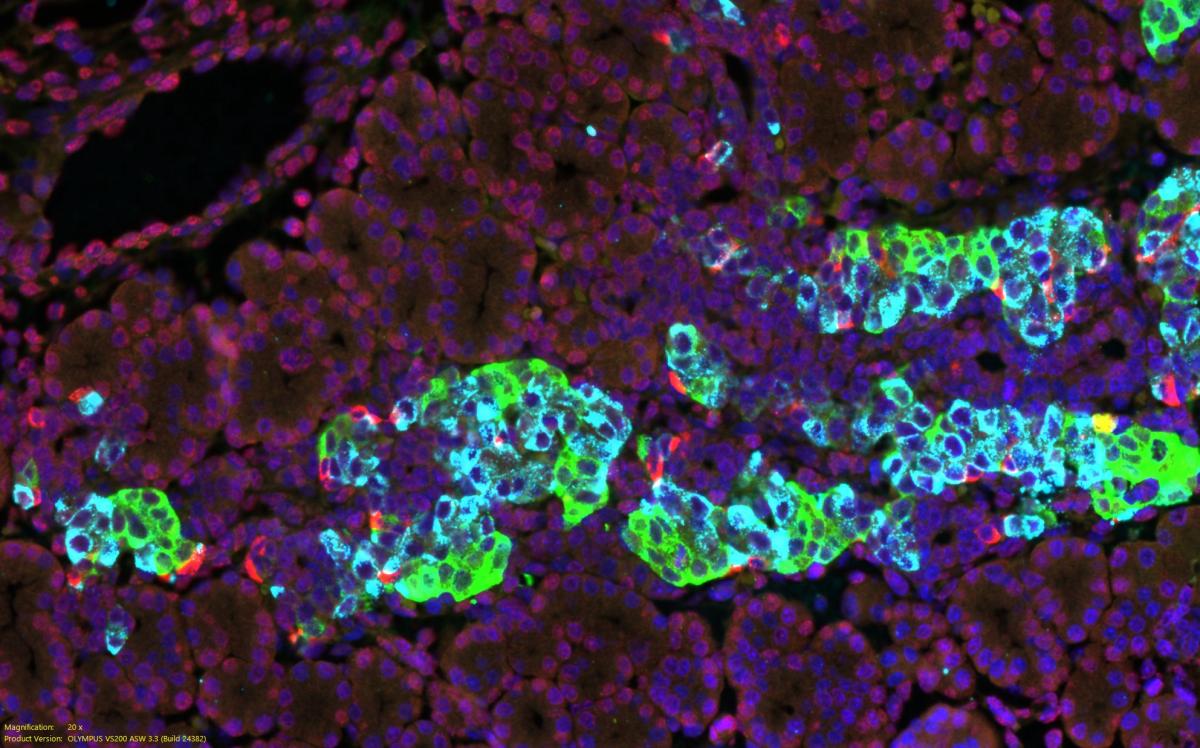
CREDIT: JI-HYEON LEE, NIDDK
Shown here are cells in an embryonic mouse pancreas and three principal endocrine hormones: Beta cells making insulin (sky blue), alpha cells making glucagon (green), and delta cells making somatostatin (red) with the fluorescent stain DAPI shown in blue identifying nuclei.
Each year, upward of 275,000 hospital stays for acute pancreatitis occur in the United States, costing 2.6 billion dollars in health care spending, according to 2018 estimates (PMID: 30315778). Experts believe that the increase is, at least in part, explained by the obesity epidemic and the increased prevalence of metabolic disease.
Why such a connection? Some of the most common causes of acute pancreatitis include gallstones and high concentrations of triglycerides, both of which are associated with metabolic syndrome. Yet alcohol use is the second leading cause following gallstones and is responsible for 25% of acute pancreatitis cases.
The powerful pancreas is a large gland behind the stomach that secretes the endocrine hormones glucagon, somatostatin, and insulin and produces enzymes that help digest food. Pancreatitis occurs when these enzymes, which can be activated by a variety of causes, damage the pancreas and lead to inflammation. Pancreatitis can be acute or chronic, and either form is serious. It can lead to complications such as nutrient malabsorption, diabetes, damage to other organs, and death.
There is no known common mechanism underlying pancreatitis, and its triggers run the gamut from trauma, surgery, certain medications, genetic predisposition, and even a scorpion sting.
Investigators from across NIH’s Intramural Research Program are using multifaceted approaches that both directly and tangentially explain the strikingly different pathways that lead to pancreatitis and ultimately inform better prevention and management. Perhaps most importantly, those scientists’ diverse work illustrates how interdisciplinary science can yield a wealth of new discoveries. Here's a sampling of some ongoing research.
Searching for genes
“You have to be a good detective in pancreatitis. The treatments are very different,” said Robert Shamburek at NHLBI’s Cardiovascular Branch. Shamburek studies rare genetic causes of lipid disorders such as hypertriglyceridemia, which often results in pancreatitis. Most of the rare genetic cases involve young children who develop pancreatitis and must be put on a nearly no-fat diet. His protocol makes use of the Clinical Center’s Genomics Opportunity (CCGO) program, which enables researchers to do whole exome sequencing of patients and sometimes their entire families. This program enabled him to identify two genetic mutations that were associated with hypertriglyceridemia.
Shamburek found that if both parents are carriers of one of those genetic mutations—an extremely rare occurrence—then their child will more than likely develop the condition.
Furthermore, by leveraging CCGO data, his team discovered that when one parent carries a mutation, if that parent becomes obese, uses alcohol, or develops diabetes, then they have about a 1-in-250 chance of developing a lipid disorder and eventually pancreatitis themselves. Shamburek hopes that identifying these rare genetic variants could inform new precision medicine prevention approaches and therapeutics.

CREDIT: NIDDK
Rebecca Brown is an endocrinologist conducting translational research. Her lab explores rare diseases to understand the basic mechanisms regulating energy metabolism in humans. Oftentimes, what is learned from treating rare diseases can be applied to improve outcomes from common health conditions, too.
Taming triglycerides
Lasker Scholar Rebecca Brown in NIDDK’s Section on Translational Diabetes and Metabolic Syndromes, Diabetes, Endocrinology, and Obesity Branch is an endocrinologist doing translational research. She studies patients with lipodystrophy, a rare disorder in which the body does not have adequate fat depots to store energy, leading to severe insulin resistance and elevated circulating triglycerides. According to Brown, upward of 20% of these patients develop acute or chronic pancreatitis. Some of those patients are deficient in the hormone leptin, which helps regulate appetite, and she treats them—often successfully—with recombinant leptin (metreleptin) (PMID: 29644599).
Despite the treatment, some patients still have persistent high triglycerides and pancreatitis. In collaboration with Shamburek, Brown published the results of a small clinical trial using volanesorsen to activate lipoprotein lipase, an enzyme that breaks down fats in the bloodstream, and showed that the drug substantially lowered triglycerides (PMID: 36195542). The mechanisms behind volanesorsen might also help other metabolic conditions.
“We’re looking at a technique to quantify uptake of fat from the bloodstream into the fat cell in patients with lipodystrophy and healthy controls,” said Brown. If successful, tracking precisely where fats are being stored could then be used in clinical trials testing drugs in common forms of obesity and diabetes.
NHLBI’s Alan Remaley, senior investigator in the Lipoprotein Metabolism Laboratory, is a pathologist who does basic science that is informing how we might treat pancreatitis in the future. Remaley’s team studies peptides that activate lipoprotein lipase. A recent paper showed how those peptides substantially helped clear triglycerides in the muscle and fat of mice (PMID: 37547254). The discovery could lead to a new treatment for the common adult forms of acute pancreatitis due to acute elevations of triglycerides. Clinical trials are likely a few years out. Read more about this work from the Lipoprotein Metabolism Lab in the Sept. 15, 2023, issue of the NIH Record.
Inflammation and autoimmunity
Inflammation and autoimmunity are yet another path to pancreatitis. Warren Strober at NIAID’s Mucosal Immunity Section studies mucosal inflammation and immune response in gastrointestinal disorders, including pancreatitis. He published a paper in 2016 on the immunopathogenesis of pancreatitis that established mechanisms demonstrating that pancreatitis is a unique form of inflammation and one that could be potentially treated with newer, more targeted therapies (PMID: 27848953). Further work by the Strober lab proposed that blocking a specific immune pathway in the autoimmune form of pancreatitis could be a successful treatment approach (PMID: 30401468).
Shmuel Muallem, senior investigator in NIDCR’s Epithelial Signaling and Transport Section, is also working the autoimmunity angle and testing new treatments. His team studies the mechanisms that govern how electrolytes such as calcium and bicarbonate are ferried across cellular membranes and what role those pathways play in autoimmune pancreatitis and Sjögren syndrome. In the pancreas, ductal cells secrete bicarbonate to neutralize acidic contents in the digestive tract, and those ducts can be damaged during disease.
Ongoing research at Muallem’s lab is exploring how drugs currently approved to treat cystic fibrosis might be repurposed to correct dysfunctional fluid secretion in mouse models of Sjögren syndrome and pancreatitis (PMID: 28634110).

CREDIT: NIAAA
Nancy Diazgranados works with patients with alcohol use disorder at the NIH CC. Alcohol is the leading cause of chronic pancreatitis, and Diazgranados advises that education about how less alcohol consumption is best for overall health should be part of the intervention strategies discussed with every patient with pancreatitis.
Pancreatitis and alcohol use disorder
“Every patient with pancreatitis should be assessed for AUD,” or alcohol use disorder, said Nancy Diazgranados, intramural deputy clinical director at NIAAA. That’s because alcohol use is the leading cause of chronic pancreatitis and second only to gallstones in acute cases. Alcohol and its metabolites can damage pancreatic tissue, and Diazgranados advises that education on how less alcohol is better should be a part of the intervention for every patient with pancreatitis.
Diazgranados conducts deep phenotyping of patients with AUD at the CC, where they undergo detox and receive counseling and multiple therapies. Researchers there collect data on behaviors, conduct neuropsychological tests and questionnaires, and do imaging and genetic testing alongside a control group without AUD. She sees three or four patients each year in her protocols who also have pancreatitis.
Treatment for pancreatitis with AUD overlaps with that for other types, that is, to temporarily stop eating, manage pain, hydrate, and then slowly resume a fat-free diet. “If it’s not treated properly, it can be fatal,” said Diazgranados, adding that for the patient with AUD, medications, psychological interventions, and support groups are important for recovery, too. She refers health care professionals to an NIAAA Core Resource on Alcohol, "Medical Complications: Common Alcohol-Related Concerns," which includes a chapter on pancreatitis and AUD to help guide clinical decision-making.

CREDIT: SUSHIL RANE
Sushil Rane is studying pancreatic pathophysiology and is investigating how fat arises in the pancreas. That understudied area could inform how we treat diseases like pancreatic cancer, diabetes, and pancreatitis.
The role of fat and neurological control
At NIDDK, Senior Investigator Sushil Rane in the Integrative Cellular Metabolism Section of the Diabetes, Endocrinology, and Obesity Branch, is known for his work on pancreatic islet development and studying how insulin-producing beta cells maintain glucose homeostasis in the body. But in recent years, his lab has taken an interest in pancreatic pathophysiology and is investigating how fat arises in the pancreas. According to Rane, it is an understudied topic that could mechanistically inform the understanding of disease areas such as pancreatic cancer, diabetes, and pancreatitis and of how we treat each because fat cells produce inflammatory cytokines and hormones that disrupt exocrine and endocrine functions.
“There is potential for fat to be made in the pancreas, but its [genetic] design suppresses that. So, is there something like inflammation or a genetic mutation that triggers this genesis of fat?” asked Rane. “Or is metabolism or another process driving fat deposition there?” His team currently has studies in the pipeline to address these questions.
Teasing out the nuanced neuroscience of pancreatic function is another fresh focus in the Rane lab. A recent study demonstrated a connection between the hypothalamus and beta cells (PMID: 35108515), and ongoing work is exploring how the vagus nerve might also control endocrine hormones. “We’re figuring out how the pancreas talks to the overall machinery in the brain and vice versa,” said Rane.
Editor’s note: The scientific discoveries happening every day at the NIH result in breakthroughs that improve human health. Across our institutes and centers (ICs), health topics such as pancreatitis are researched in a variety of ways. The NIH Catalyst hopes to reveal these multifaceted approaches to health research with a series of articles like this one. We hope these articles will help pave a catalytic path to collaboration and inspire multi-IC initiatives. If there is a health-related focus we can cover to highlight your lab’s research, please let us know by emailing us at catalyst@nih.gov.
This page was last updated on Monday, December 2, 2024
