Catalytic Research
Read about scientific advances and discoveries by NIH intramural scientists: fatigue processed differently in the brains of people with post-infectious myalgic encephalomyelitis/chronic fatigue syndrome; Omicron spike protein effectively binds to cells of the nasal passage; cognitive behavioral therapy normalized some areas of brain hyperactivation; biomarker associated with neurodegenerative disorders; reengineered cancer drug could help develop new treatments; antibody-based HIV vaccine provides protective immunity.
NINDS, NHLBI, NIDDK, CHI, NIMH, NIDCR, NIDA, NIA, NIEHS, NINR, NIAID, NCCIH, NIAMS, NCI: NIH SCIENTISTS OFFER NEW CLUES INTO CAUSES OF POST-INFECTIOUS ME/CFS
A team of multidisciplinary researchers across NIH has discovered how feelings of fatigue are processed in the brains of people with post-infectious myalgic encephalomyelitis/chronic fatigue syndrome (PI-ME/CFS).
The study took a comprehensive look at ME/CFS that developed after a viral or bacterial infection using state-of-the-art techniques to examine 17 people with PI-ME/CFS who had been sick for up to five years. They compared that cohort’s results to a cohort of 21 healthy controls. Results from functional magnetic resonance imaging (fMRI) brain scans showed that people with ME/CFS had lower activity in a brain region called the temporal-parietal junction, which may cause fatigue by disrupting the way the brain decides how to exert effort.
The researchers analyzed spinal fluid collected from participants and found abnormally low concentrations of catecholamines and other molecules that help regulate the nervous system in people with ME/CFS compared with healthy controls. Reduced concentrations of certain catecholamines were associated with worse motor performance, effort-related behaviors, and cognitive symptoms.
These findings, for the first time, suggest a link between specific abnormalities or imbalances in the brain and ME/CFS. “We think that the immune activation is affecting the brain in various ways, causing biochemical changes and downstream effects like motor, autonomic, and cardiorespiratory dysfunction,” said Avindra Nath, clinical director at NINDS and senior author of the study.
Brian Walitt, associate research physician at NINDS and first author of the study, said they may have identified a physiological focal point for fatigue in this population. “Rather than physical exhaustion or a lack of motivation, fatigue may arise from a mismatch between what someone thinks they can achieve and what their bodies perform,” he added. (NIH authors: B. Walitt, M. Hallett, S. Jacobson, Y. Enose-Akahata, P. Bedard, B. Calco, A. Gavin, D.S. Goldstein, S.G. Horovitz, K.R. Johnson, A. Jones Govan, K.M. Knutson, N. Malik, P.M. McGurrin, G. Norato, T. Popa, L.B. Reoma, F. Safavi, B. Smith, B.J. Stussman, C. Vizioli, A. Nath, K. Singh, S. Hassanzadeh, M. Levin, M.N. Sack, S.R. LaMunion, K. Chen, R.J. Brychta, A.B. Courville, R. Apps, J. Chen, F. Cheung, A. Mukherjee, B.A. Sellers, J.J. Barb, L.M.K. Chin, M.S. Deming, B. Drinkard, S. Solin, S.A. Turner, S.B. Yang, A. Williams Buckley, S. Sinclair, J. Snow, P.D. Burbelo, L.R. Feng, L. Ferrucci, R. Moaddel, S.A. Gabel, G.A. Mueller, J.D. Kreskow, L.N. Saligan, J.J. Lyons, F. Safavi, N. Madian, B.J. Stussman, A.L. Mammen, S. Muñoz-Braceras, K. Pak, I. Pinal-Fernandez, J.A. McCulloch, and G. Trinchieri. https://doi.org/10.1038/s41467-024-45107-3.)
[REPRODUCED FROM A FEB. 21 NIH PRESS RELEASE WRITTEN BY NINA LICHTENBERG, NINDS]
Learn more about this research from this NPR audio broadcast.
NCI, NIAID: UNIQUE FEATURES OF OMICRON SPIKE PROTEIN ENHANCE INFECTIVITY, IMMUNE EVASION
CREDIT: NIAID
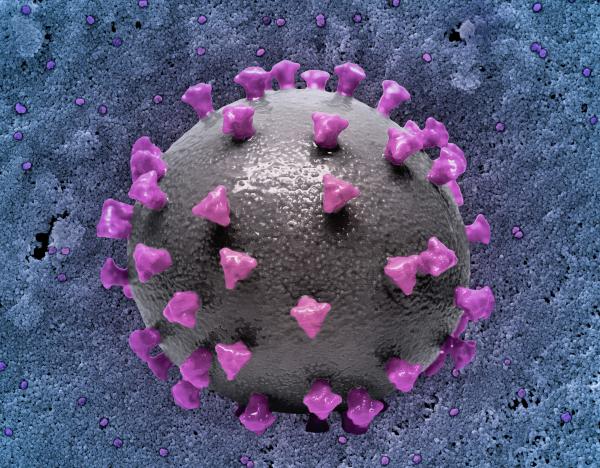
NCI, NIAID: 3D print of a SARS-CoV-2 virus particle with spike proteins shown in purple.
Researchers at NCI and NIAID recently discovered that the Omicron spike protein, which mediates viral entry into host cells, can bind to cells of the nasal passage more effectively than previous variants, including Delta, and aid in Omicron’s improved ability to infect hosts.
By creating 3D cultures of nasal epithelial cells to recapitulate conditions in the upper airway, the investigators compared several strains of SARS-CoV-2, including ancestral strains, Delta, and multiple subvariants of Omicron. They discovered a divergence in the pathways that Delta and Omicron variants used for cellular entry facilitated by their unique spike proteins. This distinctive pathway bestowed resistance to antiviral factors typically produced by host cells during infection by Omicron, highlighting a potential mechanism for immune evasion and enhanced spread.
This research adds to the understanding of the mechanisms involved in SARS-CoV-2 infection for different strain lineages, particularly the highly abundant Omicron variant, and could help guide the design of future vaccines and antiviral treatments. (NIH authors: G. Shi, T. Li, K.K. Lai, R.F. Johnson, J.W. Yewdell, and A.A. Compton, PMID: 38291024)
[BY TAYLOR FARLEY, NIAID]
NIMH: COGNITIVE BEHAVIORAL THERAPY ALTERS BRAIN ACTIVITY IN CHILDREN WITH ANXIETY
NIMH researchers discovered that cognitive behavioral therapy (CBT) normalized some areas of brain hyperactivation in children with anxiety, providing clues as to which brain regions may be associated with therapeutic responses. Pediatric anxiety disorders are common and can persist into adulthood, when they can become chronic and more difficult to treat. CBT, which helps correct dysfunctional thought processes and behaviors, is the standard treatment for pediatric anxiety.
To investigate CBT’s effectiveness in children, researchers used fMRI to compare brain activity in non-anxious children with brain activity in anxious children who received 12 weeks of CBT. Before CBT, anxious children’s brains showed hyperactivation in many regions, including areas in frontal and parietal lobes and the amygdala that help regulate cognition and emotion. CBT improved brain function in numerous overactive areas. Heightened activity persisted in others, including the amygdala, suggesting that reducing activity in those areas may require extended CBT or more targeted treatments.
Researchers also compared the children with anxiety who received CBT with a different group of untreated children at temperamental risk of developing anxiety disorders. Comparisons between those groups showed that the anxiety-associated brain activity in the at-risk group remained stable over time, demonstrating how the observed changes in the brains of children who received CBT were likely related to treatment and not the passage of time. (NIH authors: S.P. Haller, H.L. Grassie, E.L. Jones, A. Mallidi, E. Berman, K.M. Lewis, K. Kircanski, and M.A. Brotman, PMID: 38263879)
[BY KIM MORGAN, NINR]
NINDS, NIA: BIOMARKER ASSOCIATED WITH ALS AND FRONTOTEMPORAL DEMENTIA
CREDIT: NINDS
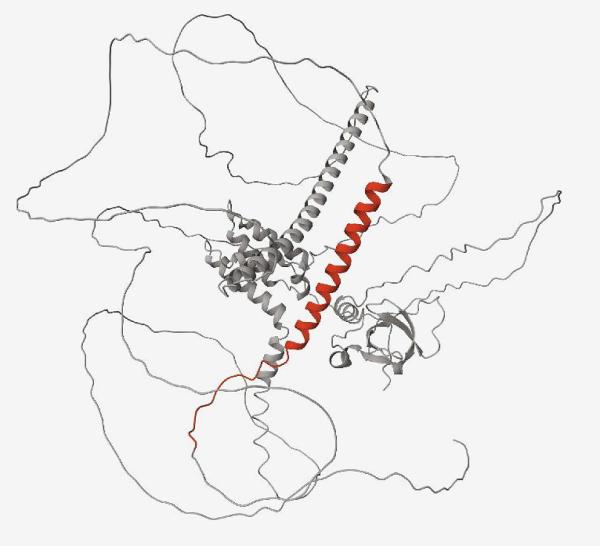
NINDS, NIA: A protein containing a cryptic peptide (red) that results from a lack of functional TDP-43, as in ALS and FTD.
A research team led by NINDS and NIA discovered abnormal proteins in the spinal fluid of people with amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD), which could serve as a biomarker for early detection of some neurodegenerative disorders.
ALS, FTD, and other similar diseases are associated with dysfunctional TDP-43, a protein that brokers proper RNA transcription. When TDP-43 malfunctions, this generates mis-spliced sections of RNA called cryptic exons, which sometimes generate abnormal proteins that are normally absent in healthy cells.
Using disease models created from induced pluripotent stem cells, the research team identified 65 proteins created from 12 cryptic exons. Next, they confirmed the presence of those proteins in postmortem brain samples from people with ALS or FTD. Then, the investigators found 18 of those new abnormal proteins in the spinal fluid of patients living with ALS or FTD.
The authors posit that the abnormal proteins could potentially trigger inflammation and lead to neurodegeneration, and in addition to serving as an early diagnostic biomarker, could also help evaluate responses to therapies in clinical trials. (NIH authors: S. Seddighi, Y.A. Qi, C. Bereda, C. Belair, A. Khandeshi, S.E. Kargbo-Hill, J. Hawrot, D.M. Ramos, H. Yuan, J. Roberts, M.A. Nalls, J.M. Colón-Mercado, J.F. Reyes, V.H. Ryan, M.P. Nelson, Z. Li, L. Screven, J.Y. Kwan, S. Jacobson, D.S. Reich, M. Maragkakis, and M.E. Ward, PMID: 38277467)
[BY ANINDITA RAY, NINDS]
NCATS, NCI: RETOOLED METHOTREXATE MAY POINT TO NEW THERAPIES FOR CANCER, AUTOIMMUNE DISORDERS
CREDIT: JAMES INGLESE, NCATS
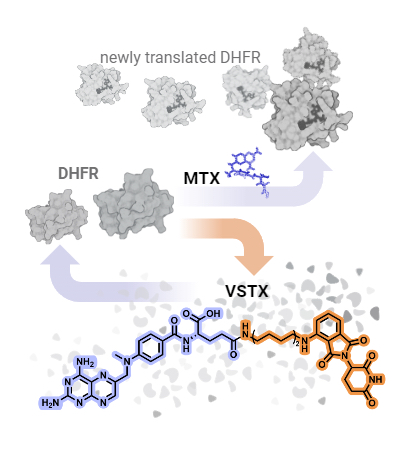
NCATS, NCI: While the presence of methotrexate (MTX) leads to greater concentrations of DHFR, versortrexate (VSTX) leads to the breakdown of DHFR.
A reengineered version of the drug methotrexate, designed by a research team led by NCATS, could lead to new tools to develop more treatments for cancer, autoimmune disorders, and rare diseases.
“This research provides scientists new tools to unravel the enigmatic properties of methotrexate and similar compounds,” James Inglese said in a press release. Inglese directs the NCATS Assay Development and Screening Technology laboratory and is corresponding author on the study.
Methotrexate is a mainstay in the treatment of blood cancers and rheumatoid arthritis. The drug blocks a protein, dihydrofolate reductase (DHFR), that plays a key role in helping cells multiply.
A translational science research team from NCATS, NCI, and the University of Oklahoma (Oklahoma City) created and investigated compounds called PROteolysis TArgeting Chimeras (PROTACs) that incorporate methotrexate. PROTACs hijack the cell’s natural waste disposal machinery to destroy a protein—in this case, DHFR. One of the new PROTACs, called versortrexate, broke down DHFR in several cancer cell lines while appearing to be less toxic than methotrexate.
Versortrexate’s very specific activity opens a range of possibilities for development. The PROTAC could be used as a chemical tool to better understand DHFR activity, and it also could reveal how similar antifolate drugs work. Combining versortrexate with a companion drug that inhibits another protein in a key biochemical pathway might prove effective at creating new antiproliferative indications.
“Molecules like methotrexate can be scaffolds for the next generation of therapeutics or chemical probes,” Inglese explained. (NIH authors: S. Rana, P. Dranchak, J.L. Dahlin, L. Lamy, W. Li, E. Oliphant, J.H. Shrimp, R. Tharakan, D.O. Holland, K.M. Wilson, S.K. Durum, D. Tao, and J. Inglese, PMID: 37875111)
[BY TERRY RUDD, NCATS]
NIAID: ANTIBODY-BASED HIV VACCINE PROVIDES PROTECTIVE IMMUNITY
CREDIT: NIAID
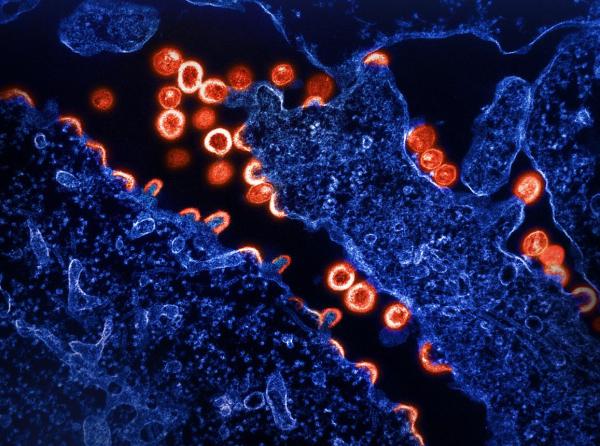
NIAID: Transmission electron micrograph of HIV-1 virus particles (red) budding and replicating from a segment of a chronically infected H9 cell (blue).
The next generation of vaccine development for human immunodeficiency virus (HIV) is being pursued at NIAID’s Vaccine Research Center (VRC). In a recent study, VRC researchers found that a novel class of vaccines delivering three different types of antibodies were each independently able to prevent and neutralize simian immunodeficiency virus (SIV), which infects non-human primates.
The three antibodies—one derived from a person with HIV and the two others from previously vaccinated rhesus macaques (Macaca mulatta)—targeted HIV’s fusion peptide, a molecular structure that allows the virus to dock with and infect a cell. Rhesus macaques are monkeys with immune systems similar to those of humans.
Animals in each of four groups received either the human-derived antibody at a high or low dose, or one of the two macaque antibodies. Five days later, the animals were challenged with a strain of SIV. All three antibodies provided statistically significant protection from SIV with the degree of protection being dose dependent.
Furthermore, breakthrough infections were seen in some of the initially protected animals in the macaque antibody groups after they underwent a SIV rechallenge a month later. When the investigators sequenced viral RNA from these reinfected animals, they found that the virus may have remained sensitive to neutralization by the infused antibody. This result suggests that the concentrations of the infused antibody in these infected animals had dropped to levels that were unable to prevent infection.
“Optimizing dosing [strategies] and broadening the neutralizing responses against HIV-1 to target its fusion peptitde would be a useful strategy for HIV vaccines,” said lead author Amarendra Pegu, who is head of antibody research at the VRC’s Virology Core. The findings could inform the design of a human vaccine. (NIH authors: A. Pegu, S.E. Lovelace, M.E. DeMouth, M.D. Cully, K. Wang, S.D. Schmidt, M. Choe, C. Liu, X. Chen, E. Viox, A. Rowshan, J.D. Taft, B. Zhang, K. Xu, H. Duan, L. Ou, J. Todd, R. Kong, N.A. Doris-Rose, P.D. Kwong, R.A. Koup, and J.R. Mascola, PMID: 38232141)
[BY DIANNE LEE]
This page was last updated on Tuesday, August 12, 2025
