NIH Hosts First-ever Industry Day
The Promise of Public–Private Partnerships
BY SEPPIDEH SAMI, CC
The new year kicked off with a renewed commitment to ensuring that research conducted at NIH translates to real-world advancements for human health.

CREDIT: NIH MEDICAL ARTS, NIAID
NIH Director Monica Bertagnolli spoke about the promise of successful collaboration between NIH scientists and private industry at NIH's inaugural Industry Day, held Jan. 18.
“When we think about delivering on NIH’s mission, we know that includes making sure that insights and discoveries that emerge from our research get out into the world and improve people’s lives,” she said, adding, “NIH does not bring new products and services to market—the agency and nation rely on productive industry partnerships to achieve this.”
Industry Day was a virtual conference that boasted 1,200 registrants, 50% of which were non-NIH, including about 30% from industry. Organizers Bibi Bielekova, chief of NIAID’s Neuroimmunological Diseases Section; Amy Klion, chief of NIAID’s Human Eosinophil Section; and Mukul Ranjan, senior advisor for Innovation and Technology Transfer at NIAID, said the event aimed to help junior scientists better understand the benefits and challenges of government–industry collaboration. At the heart of the conference were panels of scientific leaders from NIH, academia, and industry who showcased how synergistic partnerships are tackling problems at the forefront of biomedical research.
New gene therapies
Case in point: Last year, the FDA approved the first-ever gene therapy for sickle cell disease (SCD), the result of a successful NIH-industry collaboration between John Tisdale, chief of the NHLBI Cellular and Molecular Therapeutics Laboratory, and Bluebird Bio’s Melissa Bonner, who is now chief scientific officer of Nvelop Therapeutics (PMID: 35773052). Hemoglobin disorders such as SCD can be fatal and are among the most common inherited genetic diseases in the world. SCD is defined by sickle-shaped red blood cells that restrict circulation and can cause anemia, pain, and organ damage. SCD can potentially be cured with a bone marrow stem cell transplant from a healthy donor, but the procedure carries significant risk. By using a viral vector to reprogram a patient’s own stem cells to generate healthy red blood cells, the therapy eliminates the need for a matching donor and thus the risk of graft rejection.
Tisdale and Bonner shared their complex journey in bringing the treatment to market. One challenge was determining the amount of stem cells that needed to be treated to correct SCD. Another involved exhaustive genetic analyses to ensure the new genetic instructions were only reaching their intended target. Only a successful industry partnership could help overcome these challenges.
Of course, none of these studies could come to fruition without the patients who voluntarily participate in clinical trials. “They’re really our partners in bringing these transformative therapies to bear,” Tisdale said.
CREDIT: NIAID
Colorized electron micrograph of human papillomavirus (HPV) particles.
Novel vaccines
Human papillomavirus (HPV) infections can cause cervical and other types of cancers, which John Schiller, deputy chief at the NCI Laboratory of Cellular Oncology, has studied for years. Schiller spoke at Industry Day about the importance of being proactive about reaching out to industry with the discoveries made in the lab.
For example, pharmaceutical companies used Schiller’s group’s discoveries to develop and bring to market the first commercial vaccines for HPV using human papillomavirus-like particles (VLPs) (PMID: 22961341). VLPs have unique properties that also enable them to selectively bind to cancer cells and can be configured to deliver antitumor compounds. That unique ability caught the attention of Elisabet De Los Pinos at Aura Biosciences, who collaborated with Schiller to show how modified VLPs were effective and durable in treating a type of eye cancer (PMID: 29242243). The drug has moved into clinical trials, and with more research, De Los Pinos sees the potential to treat a wide range of cancers.
Better biomarkers
What if a simple blood draw could provide early detection of organ transplant rejection? That’s the idea behind analyzing cell-free DNA (cfDNA), a technique being pioneered by NHLBI Lasker Scholar Sean Agbor-Enoh. When cells are damaged, cfDNA is released into the blood and retains signatures that enable scientists to trace it back to its origin. Using cfDNA as a biomarker has shown promise in detecting lung and heart transplant rejection months before symptom onset.
Agbor-Enoh teamed with four hospitals to study the use of cfDNA instead of an invasive biopsy to monitor rejection in lung transplant patients. He credits the NHLBI Office of Technology Transfer for working seamlessly with industry partners to get the new protocol published within 18 months (PMID: 35063338). That monitoring protocol since has been adopted by multiple centers across the United States and Canada, he said.
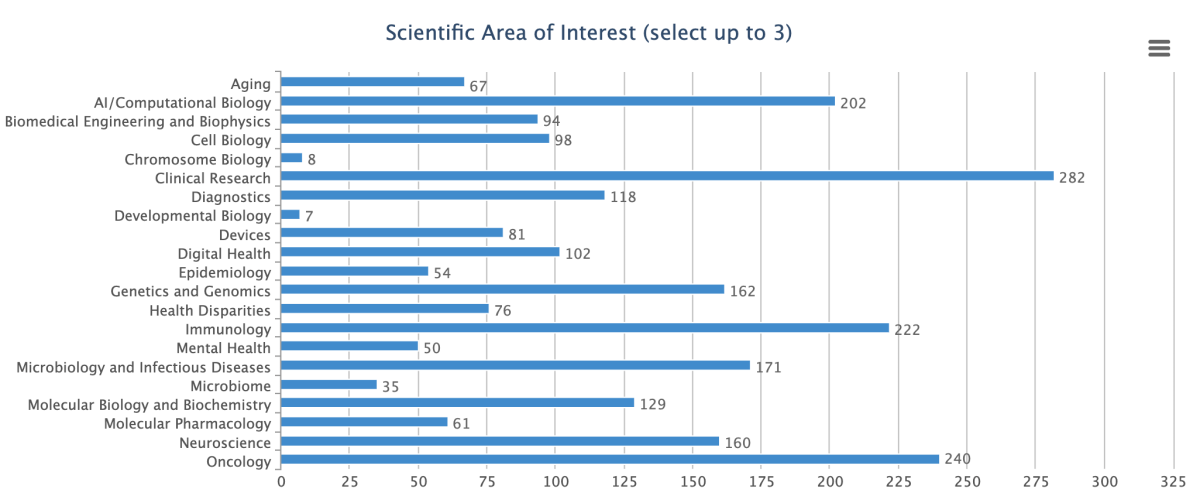
CREDIT: MUKUL RANJAN, NIAID
Shown are the results of a survey reflecting the broad scientific interest areas of the 1,200 registrants at NIH’s first-ever Industry Day.
A balanced ecosystem
Although much of Industry Day focused on productive partnerships, several speakers touted ways to improve public-private partnerships. Actionable steps included adhering to widely accepted data standards and ensuring clear goals are communicated from the outset.
“None of us works in isolation,” said Industry Day speaker Gary Nabel, founding director of NIAID’s Vaccine Research Center and, later, MODEX Therapeutics.
Organizers plan to make NIH Industry Day an annual tradition. “This was a fantastic and informative experience for the first year. I look forward to attending next year,” wrote one attendee in a post-event anonymous survey.
Seppideh Sami is a training coordinator in the Patient Support Services Department at the NIH Clinical Center. In her spare time, she enjoys studying the conservation and preservation of the natural world, especially plants.
This page was last updated on Monday, December 2, 2024
