2019 Research Festival: Plenary II
Celebrating NIH Efforts to Combat Physical and Emotional Pain
Two roads diverged in a wood, and I—
I took the one less traveled by,
And that has made all the difference.
The sentiments expressed in the Robert Frost poem “The Road Not Taken” could well describe the choices of the four presenters in their efforts to help patients dealing with physical and emotional pain.
Carlos Zarate decided to stop following the 60-year-old road of using traditional monoamine-based antidepressants that modulate serotonin, dopamine, and noradrenaline. Instead, he explored how ketamine, an anesthetic drug that blocks N-methyl-D-aspartate receptors and neuroplasticity, can be used for treatment-resistant depression. Although the traditional drugs require about six weeks to take effect, ketamine works within a single day and sometimes in a couple of hours.
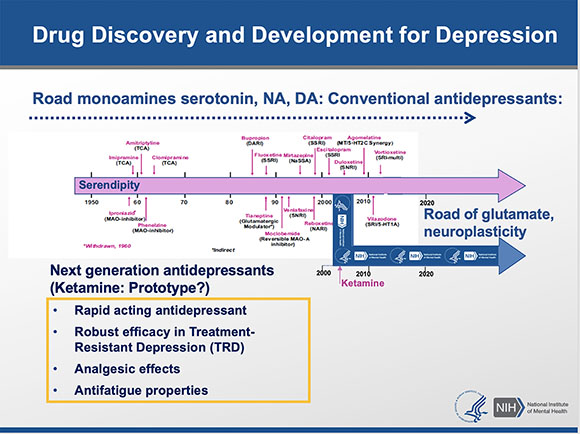
Carlos Zarate (NIMH) took a road less traveled when he veered from the traditional route of using monoamine-based antidepressants, which can take up to six weeks to treat depression. He discovered that the anesthetic drug ketamine was more effective for treatment-resistant depression and worked within hours.
But ketamine can have unpleasant side effects (high blood pressure, nausea and vomiting, hallucinations, and dissociation) and is addictive; therefore, its use is reserved for the severely depressed. Although the physiological and dissociative side effects last for only a few hours, the reduction in depressive symptoms remain for up to a week with a single administration of ketamine.
In collaboration with scientists at the National Center for Advancing Translational Sciences, the National Institute of Aging, and the University of Maryland School of Medicine (Baltimore, Maryland), Zarate and colleagues discovered some of ketamine metabolites rather than ketamine itself might be key to its rapid antidepressant effect. When the breakdown of ketamine was slowed in a mouse model, side effects occurred, and depression symptoms did not improve. If, however, the ketamine metabolite hydroxynorketamine was injected into mice, the depression symptoms improved, and there were no dissociative side effects or evidence of addiction potential. These discoveries are leading the way in finding treatments that are based on molecular mechanisms and have the potential to redefine the field of antidepressant treatment.

CREDIT: CHIA-CHI CHARLIE CHANG
During her presentation, Lauren Atlas (NCCIH, NIMH, and NIDA) described how psychological factors influence responses to pain and opioid analgesics.
Another road less traveled is one exploring the use of placebos to treat pain. Lauren Atlas (National Center for Complementary and Integrative Health) is exploring how placebos might be used instead of—or to supplement—opioid treatments. It’s known that administering placebos can reduce activity in brain regions collectively known as the “pain matrix.” Atlas, who has joint appointments with the National Institute of Mental Health and the National Institute on Drug Abuse, investigated these placebo effects further by using a balanced placebo clinical-trial design. Some patients were told that they were about to receive the drug; others were told they would not. The actual administration of the drug, however, was randomized. The patients were then exposed to pain (from heat or electric shocks in the arm, leg, or hand) while in an functional magnetic resonance imaging scanner, which measures brain activity by detecting differences in blood flow.
Atlas found that 1) there was no change in drug effects on the pain matrix whether the drug administration was hidden or open, and 2) the subjects reported that they felt less pain when they believed they were receiving the drug, regardless of whether they actually did. Interestingly, when the subjects were told they were going to receive the drug, changes were observed in the prefrontal cortex. Additional clinical studies are needed to confirm the findings.

CREDIT: CHIA-CHI CHARLIE CHANG
NIAAA Director George Koob, who is also a researcher in NIDA, invented the word “hyperkatifeia” to describe the increased negative emotional state that people experience after withdrawal from addictive drugs.
George Koob (director of the National Institute on Alcohol Abuse and Alcoholism and a researcher in the National Institute of Drug Abuse) described an unexpected path for opioids. Although opioids can relieve pain, if people become dependent on or addicted to the drugs, they can actually become hypersensitive to pain and remain so even two years after withdrawal. Koob invented the word “hyperkatifeia” to describe the increased negative emotional state experienced during withdrawal from addictive drugs. He proposed that hyperkatifeia could be caused by dysregulation of the emotional pain circuit in the brain. There are several neurotransmitters in our brain–some are designed to buffer stress, while others help in “fight or flight” scenarios. During withdrawal, the antistress neurotransmitters are dysregulated, while the stress-inducing neurotransmitters, such as corticotropin-releasing factor (CRF), are activated. This scenario causes a negative feedback cycle and enhances the likelihood of relapsing and taking the addictive drug again. Blocking CRF receptors in animal models reduced both the hypersensitivity to pain and the amount of drug consumed. By understanding the molecular circuitry behind addiction, we may be able to break the negative cycle.
Andrew Mannes (Clinical Center) explored yet another less-traveled road by considering how nonopioid therapy can be used to treat severe cancer pain. He is investigating the use of resiniferatoxin (RTX), which targets the transient receptor potential cation channel subfamily V member 1 (TRPV1) channel, also known as the capsaicin receptor, which provides a sensation of scalding heat and pain. (Capsaicin is the spicy ingredient in chili peppers.)
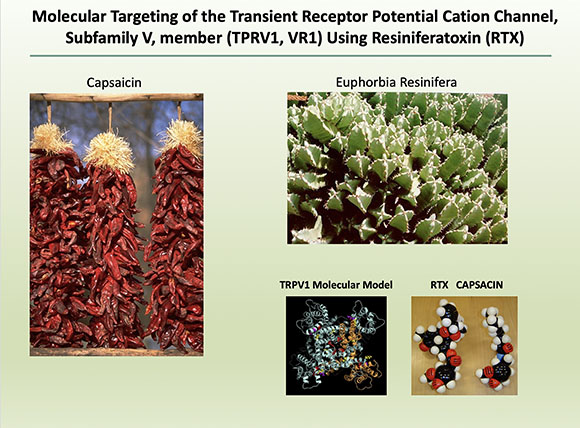
In exploring how nonopioid therapy can be used to treat severe cancer pain, Andrew Mannes has found that resiniferatoxin, which targets the capsaicin receptor, can reduce pain even when it doesn’t respond to standard pain medication. (Capsaicin is the spicy ingredient in chili peppers.)
RTX is unique in that it is highly selective for the TRPV1 channel; it is selectively expressed in a subpopulation of neurons (the A-delta fibers); and it causes the channel to be open for a prolonged time. The A-delta fibers transmit pain, and the continual opening of the channel causes an influx of calcium into the cell, resulting in cell death. This action occurs very rapidly, within a few minutes of exposure. Studies in both rats and dogs that were nonresponsive to standard pain medication revealed that RTX was efficient in decreasing the amount of pain. Mannes together with John Heiss (National Institute of Neurological Disorders and Stroke) initiated a clinical trial with cancer patients who were suffering from severe pain. One participant in the trial, who was being treated with opioids, reported a decreased pain score from 8.3 to 3.8 after RTX treatment and needed less opioid pain medication. This reduction in pain was seen in several patients in the trial. The drug did have unpleasant side effects such as urinary retention and loss of temperature sensation. Nevertheless, this clinical trial resulted in improvement of life for many participants. Further study could provide hope for patients who do not respond to traditional pain treatments.
To watch the videocast of Plenary Sessions II and III, go to https://videocast.nih.gov/launch.asp?28720.
This page was last updated on Wednesday, March 30, 2022
