2019 Research Festival: Plenary I
Celebrating NIH IRP Contributions to Curing Metabolic Diseases
The labs and clinics of the NIH intramural research program have been at the forefront of the identification and treatment of metabolic diseases. In the first plenary session of the Research Festival, four presenters, including a Nobel laureate, described their groundbreaking research.

CREDIT: CHIA-CHI CHARLIE CHANG
NHLBI alum Ferid Murad, a Nobel and Lasker laureate and NHLBI alum, talked about his discovery that nitric oxide is a signaling molecule in the cardiovascular system. The discovery led to a breakthrough treatment for cardiovascular disease and other common metabolic diseases.
The first speaker was Ferid Murad, a Nobel and Lasker laureate and alumnus of the National Heart, Lung, and Blood Institute’s intramural program (1967–1970). He shared the 1998 Nobel Prize in Physiology or Medicine with Robert F. Furchgott and Louis J. Ignarro and the 1996 Albert Lasker Basic Medical Research Award with Furchgott for their discovery that nitric oxide is a signaling molecule in the cardiovascular system. Their finding that nitric oxide relaxes smooth muscle by elevating intracellular granulocyte-monocyte progenitor (GMP) led to a breakthrough treatment for cardiovascular disease and other common metabolic diseases in the gastrointestinal tract and brain.
“We proposed that nitric oxide would be an intracellular messenger mediating the effects of various ligands and hormones,” said Murad. But this novel concept was not well accepted in the scientific community at first. “We couldn’t prove it because the concentrations required to activate [cyclic adenosine monophosphate, or cyclic GMP] were low. [T]here was no technology to measure it or its oxidation products.” The use of nitrate vasodilators was not totally new; nitroglycerin had been used for almost 100 years to treat patients with myocardial infarction, but the mechanisms behind how it worked were unknown. It took Murad nearly 10 years to convince the scientific community of the immense potential of nitric oxide as a treatment for metabolic disease.
“Resilience and persistence are really important qualities in scientists who are doing truly innovative work,” said Deputy Director for Intramural Research Michael Gottesman, who presented Murad with 2019 NIH Distinguished Alumnus Award.
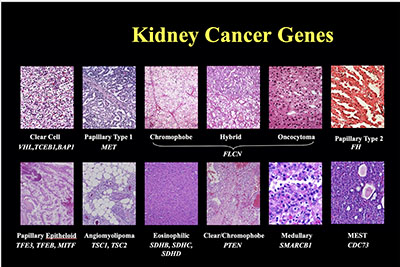
During his 35 years of research, Marston Linehan (NCI), showed that kidney cancer is not a single disease, but comes in 17 different types. He and Linehan and his colleagues also discovered genes for several different types of kidney cancer.
Resilience and persistence are certainly qualities held by the next speaker, Marston Linehan, chief of the National Cancer Institute’s Urologic Oncology Branch. During his 35 years of research, he showed that kidney cancer is not a single disease, but comes in 17 different types. Each has its own metabolic pathway and reacts differently to various concentrations of iron, oxygen, nutrients, and energy.
By studying the DNA of a large number of patients affected with familial forms of kidney cancer, Linehan and his colleagues discovered the genes for several different types of kidney cancer. They studied patients affected with von Hippel–Lindau (VHL) syndrome, a hereditary condition in which affected individuals are at risk for developing tumors in several organs including the kidneys. The first gene the group discovered was the VHL gene, which is not only responsible for VHL syndrome but for the common form of clear-cell renal-cell carcinoma as well. The VHL gene also turned out to be a critical link in understanding the body’s oxygen-sensing mechanism. The discovery of this gene and its mechanisms of action provided the foundation for the development of targeted approaches to cancer treatment and led to the FDA approval of nine drugs to treat patients with advanced kidney cancer.
Linehan and his colleagues also identified several other kidney-cancer genes that are critical for cellular nutrient and energy sensing. The research has provided a roadmap for the development of novel therapeutic approaches for papillary, chromophobe, and other types of kidney cancer.
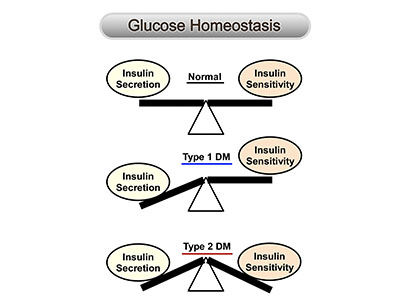
Josephine Egan (NIA) summarized her group’s progress in identifying therapeutic targets for treating type 2 diabetes mellitus.
Next, Josephine Egan summarized her group’s progress in identifying therapeutic targets for treating type 2 diabetes mellitus, a chronic condition in which the body does not produce enough insulin, a hormone that regulates blood sugar concentrations, and the effects of insulin are diminished.
Egan, who is the clinical director in the National Institute on Aging (NIA) and chief of NIA’s Diabetes Section, came to NIH in the early 1990s to study what happens to insulin secretion during aging and what role incretins (hormones that stimulate a decrease in blood glucose concentrations) may play in aging and type 2 diabetes. She found that the incretin glucagon-like peptide–1 (GLP-1), a hormone produced in the small intestine that stimulates insulin secretion and lowers blood sugar concentrations, could be used to treat type 2 diabetes. When GLP-1 is given through a continuous pump infusion for three months, insulin secretion is normalized.
This exciting discovery seemed to offer a promising treatment for people with type 2 diabetes. But there was a problem: GLP-1 is rapidly inactivated, and that’s why it needs to be given continuously. Egan’s team tested exendin-4, which is produced in the saliva of the Gila monster (Heloderma suspectum), a large lizard native to the southwestern United States. Extendin-4 is a potent agonist of the GLP-1 receptor, and it is inactivated more slowly than GLP-1. The team found that bolus injections of exendin-4 restored insulin secretion and maintained lower blood glucose concentrations in diabetic mice. The team later found that the compound increased insulin production and protected the insulin-producing cells (located in the islets of Langerhans regions of the pancreas) against damage in humans. In 2005, exendin-4 received FDA approval as a first-in-class treatment for type 2 diabetes.

In his talk, Kevin Hall explained why it’s so hard for people to maintain a healthy weight. In a clinical trial comparing ultraprocessed versus unprocessed diets, he found that people on a diet of ready-to-eat highly processed foods consumed more calories and gained an average of two pounds in a two-week period compared to people on an unprocessed diet.
The final speaker was Kevin Hall, who talked about obesity as a metabolic disease and why it’s so hard to maintain a healthy weight. Hall, who is the chief of the Integrative Physiology Section in the National Institute of Diabetes and Digestive and Kidney Diseases, studies nutrition, metabolism, and weight loss in humans at the NIH Clinical Center. He set out to challenge the common belief that reducing caloric intake by 500 kilocalories per day would result in one pound of weight loss per week.
“As you lose weight, you’re fighting an increasing battle with your slowing metabolism and increasing appetite as you lose weight,” said Hall. “You’re fighting an internal feedback-control system.” In fact, for every one kilogram of body weight lost, appetite increases above baseline by about 95 kilocalories per day and your calorie expenditure decreases by about 25 kilocalories per day.
Hall suggested it may be the quality of a diet that makes a difference, not just the number of calories or amount of carbohydrates versus fat ingested. He explained that a new method of categorizing foods, known as NOVA (which is not an acronym), classifies diets based on the proportion of calories derived from ultraprocessed foods (which often contain hydrogenated fats, high-fructose corn syrup, flavoring agents, emulsifiers, and preservatives) versus unprocessed whole foods. After conducting the first clinical trial of ultraprocessed versus unprocessed diets, Hall’s group found that, after two weeks, people on a highly processed diet consumed more calories and gained an average of two pounds, whereas people on the unprocessed diet lost about two pounds. Hall is designing a new study in which the ultraprocessed diets are being reformulated.
As the debate rages on about diets, it would it would seem to make sense to avoid, or at least reduce, the consumption of ready-to-eat ultraprocessed foods and instead eat more freshly prepared meals.
To watch the videocast of Plenary I, go to https://videocast.nih.gov/launch.asp?28719.
This page was last updated on Wednesday, March 30, 2022
