Combination Therapy Shows Increased Effectiveness Against Brain Cancer
Additional Treatment Cuts off Tumor Cells’ Escape Route From Anti-Cancer Drug
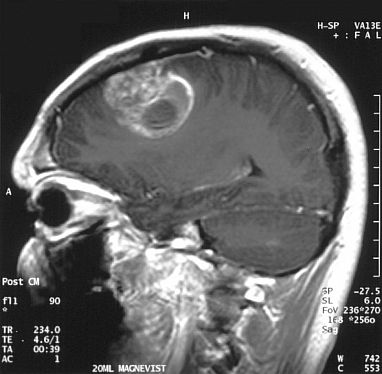
This magnetic resonance imaging (MRI) scan shows a brain tumor diagnosed as glioblastoma. New IRP research suggests a way to improve treatment for this particularly deadly form of brain cancer. Image credit: Christaras A via Wikimedia Commons
Despite the many therapies that scientists have created to fight cancer, treating the disease is often still a frustrating game of cat and mouse. Just when doctors think they have managed to defeat their wily opponent, it comes roaring back as strong as ever. A new IRP study suggests a two-pronged approach that relies in part on an existing anti-cancer drug could more effectively thwart a particularly deadly form of brain cancer.1
Unlike infectious diseases caused by bacteria or viruses, cancer results from the uncontrolled growth of our own cells. These cells can divide without constraints because of changes to cellular pathways that typically prevent them from doing so. For example, a system called the RAS pathway works abnormally in many cancers, including 90 percent of cases of glioblastoma, a form of brain cancer. While scientists have developed several drugs known as ‘MEK inhibitors’ that are effective against many tumors with changes in their RAS systems, those drugs have been largely duds in the war against glioblastoma.
“Glioblastoma is the most common malignant brain tumor, and in spite of years of study by many labs and extended, multi-model standard therapy — including surgery, radiation, and chemotherapy — median survival has increased only nominally from 8 to 10 months to between 14 and 15 months,” says IRP staff scientist Yeshavanth “Yesh” Banasavadi, the new study’s first author. “Practically, there is almost no cure.”
One of the main reasons that MEK inhibitors fail to thwart glioblastoma is that glioblastoma cells often respond to it by revving up other cellular systems to power their growth. A MEK inhibitor called trametinib, for instance, is effective against numerous types of cancer linked to changes in the RAS system but currently does not provide much benefit to glioblastoma patients because it causes their tumors to increase activity in another pathway, called the AKT pathway.
“The cells try to find an escape mechanism in order to survive, so this AKT pathway goes up,” Dr. Banasavadi explains. “Upregulation of this pathway is bad for patients but good for the tumor.”
Even when a glioblastoma tumor is surgically removed and the patient receives radiation treatment along with chemotherapy, the cancer typically recedes only temporarily.
However, prior studies led by Dr. Banasavadi before he came to NIH have shown that inhibiting a particular cellular enzyme called protein arginine methyltransferase 5 (PRMT5) not only hinders the growth of glioblastoma tumors but also suppresses the AKT pathway in them.2 Consequently, in the new study, his team and a group of collaborators from around the country investigated whether targeting PRMT5 in conjunction with trametinib treatment could stop glioblastoma cells from revving up the AKT pathway to overcome trametinib’s growth-suppressing effects.
As expected, when the researchers treated isolated clusters of glioblastoma cells with trametinib, the cells responded by increasing activity in the AKT pathway. However, when the scientists also exposed the cells to substances that either inhibited PRMT5 or reduced its levels in the cells, trametinib did not significantly bolster AKT activity. What’s more, trametinib caused more of the glioblastoma cells to die via a self-destruct process called apoptosis when they were also exposed to PRMT5-inhibiting or -reducing treatments. On the other hand, neither trametinib nor PRMT5-targeting treatments negatively affected the survival of isolated, normal human brain cells, suggesting that the treatments specifically destroy glioblastoma cells while leaving healthy cells largely intact.

Dr. Yesh Banasavadi
In a separate experiment, when mice with glioblastoma tumors in their brains were treated with only trametinib, the therapy did not increase their average survival time. However, when the mice were given a PRMT5 inhibitor along with trametinib, their survival time increased nearly 50 percent, from 19 days to 27 days on average.
While the results of the study are promising, reducing the amount of PRMT5 in glioblastoma cells is easier said than done. Just like trametinib, PRMT5 inhibitors are effective against multiple forms of cancer but currently don’t do much for glioblastoma. As a result, Dr. Banasavadi’s IRP team is working on developing ways to curb the production of PRMT5 in glioblastoma cells using a type of RNA molecule called small interfering RNA (siRNA).
“There have been multiple studies that show siRNAs, being biological agents, are a better option than using those drugs,” Dr. Banasavadi says. “In isolated cells, that’s easy to do, but in animals or humans, it’s more difficult. We are trying to come up with different strategies,” including delivering siRNAs directly to tumors via nanoparticles.
“There are multiple approaches in consideration,” he adds. “Whichever one works, it will be worth it.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Banasavadi-Siddegowda YK, Namagiri S, Otani Y, Sur H, Rivas S, Bryant J, Shelbourn A, Rock M, Chowdhury A, Lewis CT, Shimizu T, Walbridge S, Kumarasamy S, Shah AH, Lee TJ Maric D, Yan Y, Yoo JY, Jumbar SG, Heiss JD, Kaur B. Targeting protein arginine methyltransferase 5 sensitizes glioblastoma to trametinib. Neurooncol Adv. 2022 Jun 20;4(1):vdac095. doi: 10.1093/noajnl/vdac095.
[2] Banasavadi-Siddegowda YK, Russell L, Frair E, Karkhanis VA, Relation T, Yoo JY, Zhang J, Sif S, Imitola J, Baiocchi R, Kaur B. PRMT5-PTEN molecular pathway regulates senescence and self-renewal of primary glioblastoma neurosphere cells. Oncogene. 2017 Jan 12;36(2):263-274. doi: 10.1038/onc.2016.199.
Related Blog Posts
This page was last updated on Tuesday, May 23, 2023
