Comparing Two Ways to Blast Tumors
IRP Study Is Examining the Long-Term Effects of Treatments for Children With Cancer
There are several ways to use high-energy particles to kill cancer cells. IRP researchers are examining whether a particular form of radiation therapy may be a better option for children with cancer than the alternative.
Not long after German physicist Wilhelm Röntgen identified X-rays in 1895, doctors began using them to treat cancer. They soon realized, however, that this new ‘radium’ therapy — the forebearer of modern-day radiation therapy — could also cause cancer. Today, we know that radiation therapy poses much greater risks to children than adults because their cells are dividing more rapidly than those of adults, making the cells more sensitive to radiation. Children also have more years of life ahead of them during which a cancer instigated by their treatment might develop.
Despite those potential harms, radiation therapy remains a valuable tool for pediatric cancer patients, so IRP researchers are exploring the specific benefits and drawbacks of different types of radiation treatment in a new study called the Pediatric Proton and Photon Therapy Comparison Cohort.1 The project, launched in 2020 by IRP researchers in collaboration with a team at Massachusetts General Hospital, is comparing two types of radiation therapy: traditional ‘photon’ radiation therapy, which relies on the same X-rays Dr. Röntgen discovered, and ‘proton’ radiation therapy, a more advanced technology that might be less likely to trigger new cancers.
“This study has been in development for many years; it’s exciting to see it come together,” says IRP senior investigator Cari Kitahara, Ph.D., who currently leads the study. “This will be the first systematic comparison of proton versus photon radiotherapy looking at long-term cancer outcomes in childhood cancer survivors. It is a truly interdisciplinary and collaborative research effort.”

Equipment for proton therapy. The technology is costly, but may be worth the expense if it reduces pediatric patients’ risk of developing another cancer later in life.
Traditional radiation therapy shoots X-rays, which are made up of particles of light called photons, at the cancer. These photons kill cancer cells but also damage healthy cells on their way in and out of the body. In contrast, proton therapy is more targeted. Protons are electrically charged particles that pass through healthy tissue, causing less damage along the way than photons. Protons interact more and more with oppositely charged electrons as they travel through the body, progressively slowing down and delivering the most damage when they stop at the site of the tumor. Consequently, they don’t continue traveling after they reach the tumor, unlike photons. This enables them to be used for radiation therapy without causing as much damage to non-tumor tissue.
Over the last decade, proton therapy’s tumor-targeting perks have increased its popularity, especially among doctors who treat children with cancer. The number of children treated with proton therapy doubled from 2012 to 2016, and support continues to grow. Still, definitive evidence showing that proton therapy works better and is less harmful than photon therapy remains sparse because the question is hard to study, especially in children.
“There’s never been a randomized trial that tests the benefits of protons versus photons in children for preventing subsequent cancers,” Dr. Kitahara says. “It’s assumed that protons have these greater benefits compared to photons, so a trial would be considered unethical. There have been observational studies, but they have significant limitations.”

IRP senior investigator Cari Kitahara leads the Pediatric Proton and Photon Therapy Cohort study.
The Pediatric Proton and Photon Therapy Comparison Cohort has been designed to overcome those limitations and reduce biases seen in previous studies. Funded in part by the Childhood Cancer Data Initiative run by NIH’s National Cancer Institute (NCI), the study is collecting data from 20,000 childhood cancer patients treated at participating cancer centers: 10,000 who received proton therapy and 10,000 who received photon therapy between 2007 and 2023. This large sample size is essential for answering the key question of whether proton therapy reduces the risk of second cancers in childhood cancer survivors compared to photon therapy. Second cancers occur in about 2 percent of childhood cancer survivors within the first 15 years after treatment, which means that researchers need to look at a lot of survivors to find enough second cancers to detect a statistically significant difference between the two types of treatment.
As of August 2024, the study had recruited more than 7,200 patients who had been treated at 13 cancer centers across the United States and Canada. About half of these patients were treated before age 10, and most were treated for a brain or central nervous system tumor. Participating cancer centers provide researchers with patient demographic information, clinical data related to their diagnosis, and detailed radiation therapy plans. Here, the study’s specialized team of medical physicists plays an important role.
“The NCI dosimetry team, led by Dr. Choonsik Lee, has developed very sophisticated methods to use radiation therapy plans to calculate how much radiation the tumor and other organs and tissues have absorbed,” Dr. Kitahara says. Knowing that, she adds, will help determine whether higher doses of either type of radiation are linked to a higher risk of second cancers.
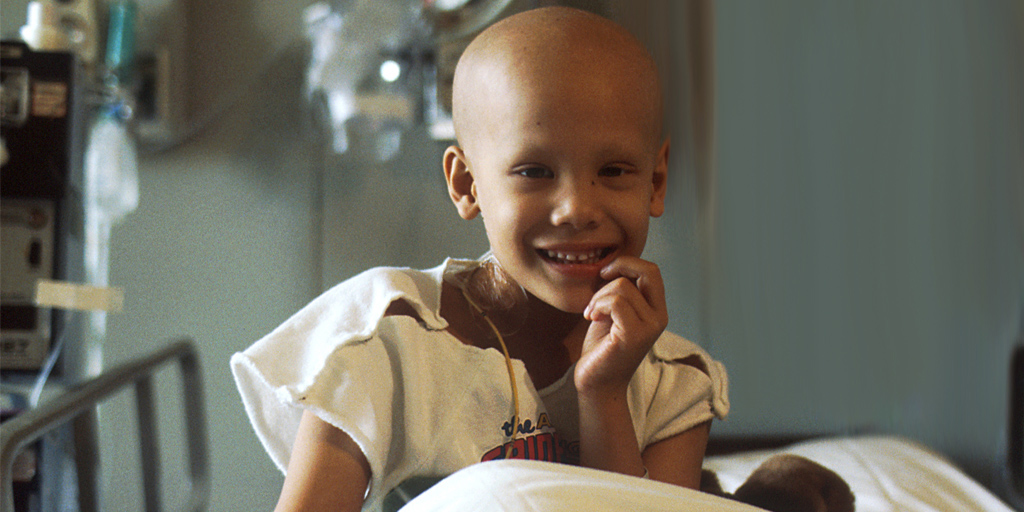
The study will track outcomes for thousands of children with cancer being treated across the United States and Canada.
To find out which patients develop a second cancer, the researchers will link medical records to state and provincial cancer registry data. Linking to registries, instead of relying on follow-up data from the cancer centers, will help the researchers find as many second cancers as possible.
The use of those registries, in addition to the study’s large sample size and precise measurement of radiation doses, helps ensure that any differences between photon and proton therapy that the study identifies truly exist rather than being due to chance. Even so, Dr. Kitahara says it will be many years before the team has data on second cancer outcomes. However, insights into whether protons are truly less harmful than photons to healthy surrounding tissue could arrive much sooner from Dr. Lee’s team, as they estimate and compare radiation doses from both treatment types.
Data from the study could not only inform doctors’ treatment decisions and follow-up care for childhood cancer survivors, but also justify investment in costly proton beam technology. After all, if proton therapy keeps childhood cancer survivors from enduring a second bout with the disease, any upfront costs will be well worth it.
“It’s clear from our experience developing and building this cohort that the clinical community is eager to work with NCI and the Childhood Cancer Data Initiative to achieve the goal of improving cancer treatments,” Dr. Kitahara says. “There’s this collective excitement around this study, and that’s really rewarding.”
References:
[1] Berrington de Gonzalez A, Gibson T, Lee C, et al. The Pediatric Proton and Photon Therapy Comparison Cohort: study design for a multicenter retrospective cohort to investigate subsequent cancers after pediatric radiation therapy. Adv Radiat Oncol. 2023 Nov 23;8(6):101273. doi: 10.1016/j.adro.2023.101273.
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
Related Blog Posts
This page was last updated on Wednesday, September 4, 2024
