Targeting Tumors in the Brain
IRP Research Brings Hope to Patients with Deadly Cancer
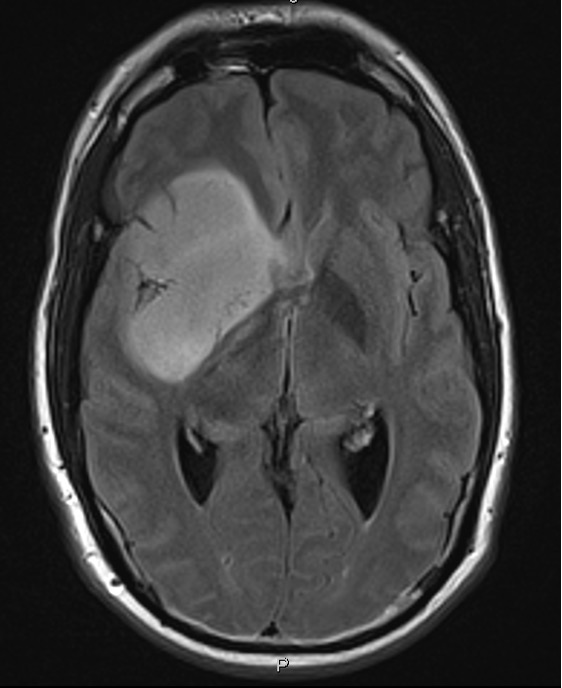
IRP Lasker Clinical Research Scholar Jing Wu is working to improve treatment for brain tumors called gliomas, pictured here.
Among the many forms of brain cancer, glioma may be the most well-known, having recently taken the lives of Ted Kennedy in 2009, John McCain in 2018, and actor Tim Conway in 2019. Despite the attention drawn to it by the deaths of these public figures, glioma remains both mysterious and highly lethal. Fortunately, IRP researchers are fighting back against this stubborn foe. In preparation for World Brain Day on July 22, we talked with IRP Lasker Clinical Research Scholar Jing Wu, M.D., Ph.D., about her efforts to better understand glioma and identify potential therapies to treat the deadly disease.
Glioma is an umbrella term for cancers that form in nervous system cells called glia. They are sometimes called the glue that holds the nervous system together, providing the physical and chemical support neurons need to function. Gliomas are the most common type of cancerous tumor originating in the brain, and they are also the most aggressive and difficult to treat. Rarely fully curable, most of the patients diagnosed with glioma die from the disease.
One reason that gliomas are so difficult to treat is that they are highly diverse, carrying a large variety of genetic mutations and molecular mishaps. This makes them both more aggressive and more likely to develop resistance to therapies. However, if the genetic makeup of a tumor can be identified, it may be possible to choose a more targeted therapy with a better chance of success.
“Many tumors have unique features that make them vulnerable to certain treatments,” says Dr. Wu. “By understanding the biology of the tumor, you can find its weakness and target it.”
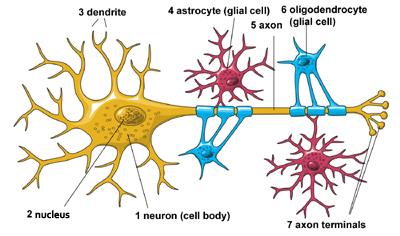
Neurons in the brain depend on supportive cells, called glia or glial cells, in order to survive and function properly. There are multiple types of glia, including oligodendrocytes and star-shaped astrocytes.
Dr. Wu's lab in the Neuro-Oncology Branch of the National Cancer Institute (NCI) is tackling gliomas from two angles: better treatment and better diagnosis. Much of her work currently focuses on a specific mutation that affects an enzyme called isocitrate dehydrogenase (IDH), which is involved in the chemical reactions needed to release energy from the food we consume. Gliomas with a mutation in the IDH gene are a common form of adult brain cancer among patients under 50.
“The IDH mutation causes unique characteristics in the glioma, so it behaves differently from tumors without the mutation,” says Dr. Wu. “The affected tumors have features that make them vulnerable to certain treatments, which means doctors can lower the dose and cause less toxicity for the patient.”
When she first came to NIH in 2015, Dr. Wu and her postdoctoral fellow, Yu-Ting Su, Ph.D., began studying a drug called zotiraciclib, which had been used to treat other cancers, but not ones in the brain. The two scientists discovered biological effects of the drug that hadn’t been described previously, which hinted that the drug might be helpful for fighting gliomas. 1 As a result, they decided to test it in patients, launching a clinical trial2 that ultimately spurred the U.S. Food and Drug Administration (FDA) to grant zotiraciclib an ‘orphan drug’ designation as a treatment for all gliomas, which is not the same as an FDA approval but does provide incentives for pharmaceutical companies to develop the drug into a treatment.
However, Dr. Wu’s team wasn’t done. When the group delved deeper into the data from the trial, they found something unexpected: it appeared that the drug worked particularly well in patients whose tumors had the IDH mutation. Further laboratory experiments using cells grown from glioma patients’ tumors confirmed that suspicion.
Random genetic mutations can cause cells to become cancerous, but those same mutations may also make cancers particularly vulnerable to specific therapies.
At the same time, Dr. Wu’s team approached the question from the other direction, working with the National Center for Advancing Translational Sciences (NCATS), to test the effects of over 2,400 different drugs on a group of patient-derived glioma cells with IDH mutations to see which ones could effectively kill the cancer cells while also having a good chance of being able to penetrate the blood-brain barrier, which often prevents therapies for brain cancer from reaching tumors.
“Zotiraciclib was one of the top hits, which strengthens our rationale for developing it as a treatment for IDH-mutant gliomas,” says Dr. Wu. “It gave us independent evidence to support testing it again in patients with this particular type of glioma.”
Encouraged by that discovery, Dr. Wu’s team once again took their bench discoveries to the bedside by developing a clinical trial testing zotiraciclib specifically in patients whose gliomas have IDH mutations. That clinical trial is currently open to patient enrollment.
Dr. Wu’s lab is also conducting additional studies to determine how gliomas become resistant to zotiraciclib over time in the hopes of finding a way to prevent that. Most likely, she says, doctors will end up using the drug in combination with another treatment for a synergistic effect that lessens the odds that the tumor will come back after initially responding to treatment.
Along with improving treatment for glioma, Dr. Wu is developing an imaging method that could be used for non-invasive diagnostics. Her approach is called metabolic imaging, a type of magnetic resonance imaging (MRI) that allows specialists to look at metabolites, the chemical components needed to make energy. This allows scientists to assess the molecular events going on inside a tumor, as opposed to the structural elements that traditional MRIs focus on. Dr. Wu is currently planning a first-in-human clinical trial to test the safety of this metabolic imaging approach in glioma patients with IDH mutations.

Dr. Jing Wu
“Building clinical metabolic imaging is a long process, but it’s worth it because it’s unique for brain tumors,” Dr. Wu says. “You can’t take multiple biopsies of a tumor in the brain the way you can with skin cancer, so we need a non-invasive tool to monitor the patient’s disease and their response to the treatment.”
Tumors of different types and at different stages of disease have unique metabolic patterns, so Dr. Wu’s team is hoping to use the approach to identify differences between tumors that respond to treatment and tumors that remain undeterred by therapy. By comparing their imaging results with traditional methods of examining tumors — like taking samples of tissue with an invasive biopsy — the IRP researchers aim to develop an easier, less invasive way to monitor the disease and gauge a tumor’s response to treatment.
“Basically, we are building the infrastructure for the clinical program to monitor disease and treatment response for brain tumor patients,” Dr. Wu explains.
Bridging her laboratory work to her clinical work makes Dr. Wu’s role in the Intramural Research Program her “dream job,” she says. In fact, she moved her office into her lab space, which allows her to see patients in close proximity to her postdocs and the activities going on at the laboratory bench. She makes sure to take time to communicate with patients about her lab’s research advances, and she even sometimes takes patients into her laboratory so they can see first-hand how their blood and tissue samples are being used to understand their disease and seek effective treatments.
“Conducting research gives me a sense that I’m doing something for the patient,” she says. “We get them involved so they have a sense of how they are contributing. Even though the study result may not help them directly, they know it’s helping the entire community. And it helps keep the researchers in the laboratory very motivated, which is important because they don’t usually get to see what is happening in the clinical world. Once they put a name to the patient, they will know this isn’t just a test tube of blood, it’s someone’s life.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Su YT, Chen R, Wang HR, Song H, Lappin H, Vasconcelos G, Lita A, Maric D, Celiku O, Li AG, Zhang W, Larion M, Abu-Asab M, Zhuang P,Yang CZ, Gilbert MR, Wu J. Novel Targeting of Transcription and Metabolism in Glioblastoma. Clinical Cancer Research 2018 Mar 1; 24(5):1124-1137. doi: 10.1158/1078-0432.CCR-17-2032.
[2] Wu J, Yuan Y, Long Priel DA, Fink D, Peer CJ, Sissung TM, Su YT, Pang Y, Yu G, Butler MK, Mendoza TR, Vera E, Ahmad S, Bryla C, Lindsley M, Grajkowska E, Mentges K, Boris L, Antony R, Garren N, Siegel C, Lollo N, Cordova C, Aboud O, Theeler BJ, Burton EM, Penas-Prado M, Leeper H, Gonzales J, Armstrong TS, Calvo KR, Figg WD, Kuhns DB, Gallin JI, Gilbert MR. Phase I Study of Zotiraciclib in Combination with Temozolomide for Patients with Recurrent High-grade Astrocytomas. Clin Cancer Res. 2021 Jun 15;27(12):3298-3306. doi: 10.1158/1078-0432.CCR-20-4730.
Related Blog Posts
This page was last updated on Thursday, July 20, 2023
