IRP’s Harvey Alter Awarded Nobel Prize
NIH Researcher’s Pioneering Work Led to Discovery of Hepatitis C

NIH’s Dr. Harvey Alter won the 2020 Nobel Prize in Physiology or Medicine for his life-saving discoveries about hepatitis C.
When the phone rang at 4:15 in the morning, IRP senior scientist Harvey J. Alter, M.D., was annoyed. He didn’t answer it. After the third try, he reluctantly got out of bed and took his phone out to the hallway.
“Before I could yell at the person, he said, ‘This is Stockholm calling,’” Dr. Alter recalls. “And then I got stopped in my tracks. Then the moment of disbelief and awe comes over you.”
The man from Stockholm informed Dr. Alter that he had won the 2020 Nobel Prize in Physiology or Medicine for his contributions to the discovery of the hepatitis C virus. He shared the prize with Michael Houghton, Ph.D., of the University of Alberta, Canada, and Charles M. Rice, Ph.D., of Rockefeller University in New York.
Hepatitis is an infectious disease that affects the liver and causes stomach pain, yellowing of the eyes and skin, and fatigue. In serious cases, it can lead to a form of liver damage called cirrhosis, as well as liver cancer and liver failure. There are five types of hepatitis — A, B, C, D, and E — that differ in their severity, methods of transmission, and where they are commonly found. The NIH’s National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) estimates that between 2.7 and 3.9 million people in the U.S. have hepatitis C, and because it can exist in the body without symptoms for a long time, many people don’t know that they have it. As opposed to both hepatitis A and B, there is no preventive vaccine for hepatitis C, and effective antiviral treatments for hepatitis C only became available in 2013.
Doctors first described hepatitis A and B in the 1940s. Hepatitis A is temporary and spreads through contaminated food and water, whereas hepatitis B can persist in the body indefinitely and spreads through bodily fluids like blood. No one knew there were more forms of hepatitis until Dr. Alter and colleagues at the NIH encountered a puzzling mystery: some patients who received blood that tested negative for hepatitis A and B still came down with hepatitis.

More From the IRP
Blog
A Long Tradition of Vaccine Breakthroughs
Dr. Alter joined the NIH as a clinical associate in 1961, fresh from completing an internship at Rochester University’s Strong Memorial Hospital in New York. He was working in hematology at the NIH Clinical Center’s blood bank when a colleague suggested he connect with future Nobel laureate Baruch Blumberg, M.D., Ph.D., who was studying the role that inherited differences in blood proteins have on a person’s susceptibility to different diseases.
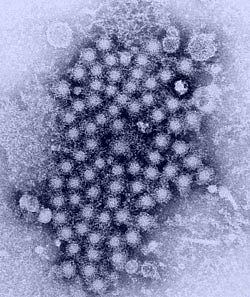
Image credit: U.S. Centers for Disease Control and Prevention
A cluster of hepatitis viruses, as seen through a transmission electron microscope.
That connection turned out to be the beginning of a fateful collaboration. In the course of their experiments, the pair discovered a protein that, several years later, turned out to be the molecule that triggers the body to produce antibodies against hepatitis B. The discovery of this protein, which they initially called the ‘Australia antigen’ after the blood sample donor’s home country, eventually led to the isolation of the hepatitis B virus and enabled the creation of testing methods for hepatitis B in patients and donated blood.
However, while this development dramatically reduced the number of people infected with hepatitis B through blood transfusions, Dr. Alter and his team showed in the mid-1970s that a large number of patients who received blood still developed hepatitis. Even more curious, tests found neither hepatitis A nor B were the culprit, setting off a long, arduous search for a mysterious third strain of the virus. For more than a decade, researchers employed every technique available to isolate the virus, without success.
“It was the early days of molecular biology,” Dr. Alter explains. “Cloning a virus is relatively simple to do now — COVID-19 was cloned within a week of its discovery — but it took 15 years to identify hepatitis C.”
In 1986, Nobel Prize co-winner Michael Houghton and his colleagues at the pharmaceutical company Chiron managed to piece together several DNA fragments, some of which contained the virus. They ultimately named this virus hepatitis C, and they also identified a critical viral protein and developed a method to detect antibodies that bind to it. Subsequently, Dr. Alter’s lab at the NIH showed that 90 percent of patients who developed hepatitis not caused by the hepatitis A or B viruses tested negative for that antibody before receiving a blood transfusion but positive weeks after the transfusion. This confirmed that the non-A, non-B variety of hepatitis Dr. Alter’s team was investigating was the same virus that Dr. Houghton and the Chiron scientists were studying.

Dr. Harvey Alter (left) and Dr. Robert Purcell (right), pictured in the Blood Bank of the NIH Clinical Center, where they worked together in the late 1980’s to identify the virus that causes hepatitis C.
What’s more, Dr. Alter’s team found that nearly 90 percent of patients infected with hepatitis C received blood from a donor who was infected with the virus but showed no symptoms. This suggested that routine testing of blood donors for antibodies to hepatitis C might prevent a large number of cases of transfusion-associated hepatitis, and this testing was implemented nationwide in 1990. As a result, transfusion-associated hepatitis was virtually eliminated by 1997, thereby preventing millions of cases in the US over the next two decades. What’s more, the discovery of hepatitis C led to the development of drugs that are now almost 100 percent effective at curing the disease.
“Seeing the first patient identified as having non-A, non-B hepatitis and then seeing the near eradication of transfusion-associated hepatitis and the ability to cure most chronic hepatitis C carriers has been extremely gratifying and was not anticipated when these studies began,” says Dr. Alter.
Dr. Alter credits the culture of NIH for his success, from the many mentors and colleagues he has worked with to the steady support for his years of painstaking, incremental research.
“I don’t have a Ph.D. and I’m not trained in laboratory science but, over the years, I collaborated with scientists such as Robert Purcell and Patrizia Farci who are so smart and so adept at research, and I fed off of them,” says Dr. Alter. “Basically, I’m a parasite, but in a good way.”
COVID-19 has disrupted the Nobel ceremony along with everything else, so instead of making the traditional trip to Sweden, Dr. Alter collected his medal and filmed his Nobel lecture in the NIH Clinical Center’s Lipsett Auditorium.
“I owe everything to the NIH and the Clinical Center,” he says. “That’s the room where it happened, and I’m ever grateful for that.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program. Learn more about other IRP scientists who have been awarded the Nobel Prize.
Related Blog Posts
This page was last updated on Monday, January 29, 2024
