Lab-Designed Virus Shows Promise for Inner Ear Gene Therapy
Delivery Method Could Eventually Help Correct Mutations That Cause Hearing Loss
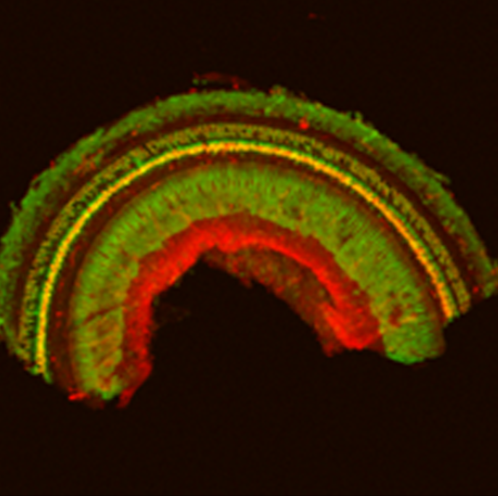
IRP scientists have shown that a particular virus can effectively deliver a gene that produces a glowing green protein to multiple cell types throughout the inner ear (above), making the virus a promising tool for gene therapy in individuals with hereditary hearing loss.
Most people probably think of viruses as villains that bring illnesses like measles, HIV, and the flu, but some viruses are proving to be valuable allies in the fight against genetic diseases. In a new study, a team of scientists from the NIH IRP and their colleagues showed the promise of a lab-designed virus for delivering gene therapies aimed at correcting hereditary hearing loss.1
Hearing loss, one of the most common disabilities globally, can be caused by aging, exposure to loud noise, or genetic mutations that impair or kill the ear’s sensory cells, called hair cells. While hearing can be partially restored with a hearing aid or a device called a cochlear implant, these gadgets do not perfectly replicate true human hearing. As a result, many scientists are trying to develop ‘gene therapy’ techniques to directly fix the faulty genes that cause hereditary hearing loss.
“With the gene therapy approach you can potentially go in to the affected cells and actually fix the underlying problem,” says IRP principal investigator and Johns Hopkins School of Medicine associate professor Wade Chien, M.D., the new study’s senior author. “If you have a mutation in a gene so that the inner ear is not making the product of that gene, we could deliver normal copies of that gene into the inner ear to restore the production of that gene’s product and repair hearing.”
Most current gene therapy research relies on viruses to deliver new genes to cells because viruses naturally infect cells by injecting their own genetic material into them. A type of virus called adeno-associated virus (AAV) is one of the most commonly used viruses in gene therapy research because it does not harm humans. However, naturally occurring AAVs have significant limitations when it comes to gene therapy for hearing loss: many of them are good at infecting only one of the two types of sensory cell that are critical for hearing, called inner hair cells, but not the other, called outer hair cells.
In the new study, Dr. Chien’s team tested the ability of several AAVs – some naturally occurring and some created in other research labs – to deliver genes to the several types of cells in the ears of mice. Rather than introducing a gene related to hearing, the viruses instead inserted a gene that produces a glowing protein, making it easy for the researchers to see which cells were infected by the virus.
While all of the viruses tested could infect both inner and outer hair cells to varying degrees, a lab-designed virus called AAV2.7m8 showed the most promise, delivering the gene to over 80 percent of both cell types. It also infected hair cells in locations throughout the ear.
“The infection efficiency is really critical for a good result,” Dr. Chien explains. “In the past, we have done studies where we injected a certain gene therapy into a mouse model of hearing loss, and we found that there was no hearing recovery because the infection rate was low, but if we increased the infection rate, often times we started to see some functional recovery.”
The AAV2.7m8 virus was also adept at infecting two types of supporting cells in the ear that are an additional promising target of gene therapy for hearing loss because they are thought to promote the growth of new hair cells when old ones die.2 Moreover, AAV2.7m8 had no adverse effect on the hearing or balance of the mice, suggesting that it is likely to be safe for use in humans because it did not harm the cells in the ear that support those functions. Nevertheless, before human testing can begin, scientists must test the virus’s ability to deliver gene therapy to the ears of mouse and other animal models of hearing loss.
“We are very excited about this virus,” Dr. Chien says. “I think it could be a very powerful and useful virus for investigators interested in using gene therapy to improve hearing in individuals with hearing loss.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] AAV2.7m8 is a powerful viral vector for inner ear gene therapy. Isgrig K, McDougald DS, Zhu J, Wang HJ, Bennett J, Chien WW. Nat Commun. 2019 Jan 25;10(1):427. doi: 10.1038/s41467-018-08243-1.
[2] Generation of hair cells in neonatal mice by β-catenin overexpression in Lgr5-positive cochlear progenitors. Shi F, Hu L, Edge AS. Proc Natl Acad Sci USA. 2013 Aug 20;110(34):13851-6. doi: 10.1073/pnas.1219952110. Epub 2013 Aug 5.
Related Blog Posts
This page was last updated on Wednesday, May 24, 2023
