Mosquitos With Human Gene Hinder Malaria Transmission
Genetically Modified Insects Could Help Curb Infections

Photo courtesy of Dr. Joel Vega-Rodriguez
IRP researchers gave mosquitos a human gene, called PAI-1, to reduce the bugs’ ability to transmit malaria. The fluorescent color in their eyes is used as a marker to distinguish the different varieties of genetically modified mosquitoes from one another and from their genetically typical counterparts.
“Scientists create genetically modified mosquitos” sounds like the plot of a bad sci-fi movie, but it’s actually the reality in labs all around the world. Researchers are producing these ‘transgenic’ mosquitos in the hopes that the bugs could help combat the scourge of malaria, and in a recent study, IRP scientists demonstrated that their unique strategy in this realm has strong potential to accomplish that goal.1
According to the US Centers for Disease Control and Prevention (CDC), nearly half of the world’s population lives in areas where malaria is common. The disease, which occurs when an infected mosquito’s bite introduces Plasmodium parasites into a person’s body, caused more than 240 million illnesses and 600,000 deaths in 2020. The disease mostly affects children and pregnant women living in Africa.
While bed nets treated with insecticides and antimalarial drugs have helped to dramatically reduce the number of malaria cases and deaths, this drop has plateaued in recent years as mosquitos have become resistant to the insecticides and the Plasmodium parasite has adapted to shrug off antimalarial medications. Moreover, existing vaccines for the illness only offer protection for a few months at a time, and going back to a clinic for repeated vaccine doses is difficult in the many remote areas of Africa and Asia where malaria is common.
“Malaria has been with us for thousands of years,” says IRP Stadtman Investigator Joel Vega-Rodriguez, Ph.D., the new study’s senior author. “If you go back in history, you can find references to malaria from the ancient Mesopotamian and Greek time. It’s been with us for a long time, and we haven’t been able to control it.”

Dr. Joel Vega-Rodriguez
As the fight against malaria continues, modern scientists are now manipulating mosquito DNA to produce insects that could be introduced in malaria-prone areas to help control the disease’s spread. Some of these bugs have a super-charged immune response to the Plasmodium parasite, while others produce antimalarial molecules in their bodies. Dr. Vega-Rodriguez’s lab, on the other hand, is one of very few — and possibly the only one — that has given mosquitos a human gene to curtail their ability to spread malaria.
Dr. Vega-Rodriguez’s team began pursuing this approach after it discovered that the Plasmodium parasite needs to coat itself with a protein found in the human body, called plasminogen, in order to reproduce inside mosquitos and pass from them to humans.2 When plasminogen is converted into its active form, it degrades a molecule called fibrin that forms blood clots, as well as proteins in the ‘extracellular matrix,’ a molecular mesh that surrounds and protects our cells and tissues. This allows the parasite to move around and reproduce in the mosquito’s gut, which is full of clotted blood, and it also enables the parasite to enter our bodies by tunneling through the extracellular matrix of our skin after an infected mosquito bites us.
“Imagine the parasite as having scissors or a machete and just chopping its way through these extracellular matrix proteins that it has to traverse in order to find a blood vessel,” Dr. Vega Rodriguez explains.
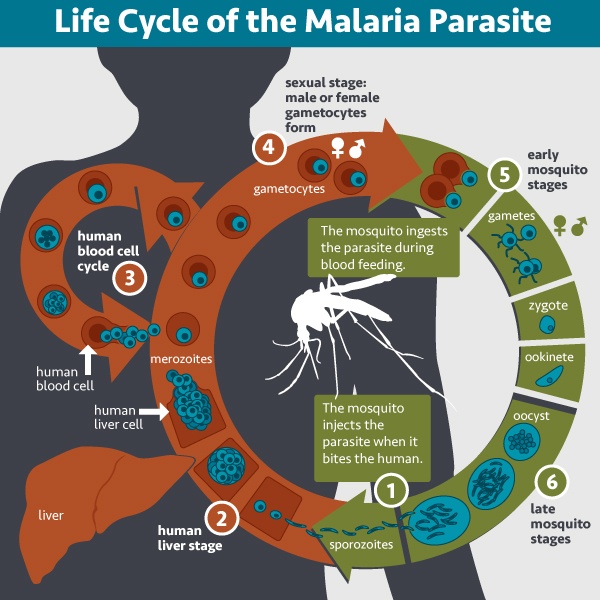
This diagram shows the lifecycle of the Plasmodium parasite that causes malaria.
To create mosquitos that are less likely to spread malaria, his team gave the bugs a human gene called PAI-1, which produces a protein of the same name that prevents plasminogen from being activated. Stopping plasminogen activation, the researchers reasoned, would rob the Plasmodium parasite of the molecular machete it needs to reproduce and spread from mosquitos to humans. Indeed, in their new study, they found that mosquitos that ingested blood containing the parasite and PAI-1 had much less of the parasite’s reproductive form, called an oocyst, in their guts a week later. However, when the active form of plasminogen was added to the blood, the parasite readily multiplied inside the mosquitos.
The IRP team went on to create genetically modified mosquitos that produced PAI-1 in their guts, their salivary glands, or both. The genetic alteration did not reduce the length of the mosquito’s lives or their ability to ingest blood or reproduce, but it did have a dramatic effect on their potential to spread malaria. All three varieties of genetically modified mosquito had significantly fewer oocysts in their guts, and their salivary glands contained much lower numbers of Plasmodium sporozoites, the mobile, infectious form of the parasite that develops inside the oocysts. This reduction was due not only to the smaller number of oocysts in the modified mosquito’s guts, but also because PAI-1 production altered the structure of the mosquitos' salivary glands in a way that made it more difficult for the sporozoites to infiltrate them.
“We knew that the number of oocysts in the gut was reduced, but there were still plenty of them, and each one produces thousands of sporozoites, so we expected to see good salivary gland invasion,” Dr. Vega-Rodriguez says, “but when we actually looked at the number of sporozoites in the salivary gland, we saw there were very few, which suggests there’s an additional barrier. That makes life for the parasite that much more difficult.”
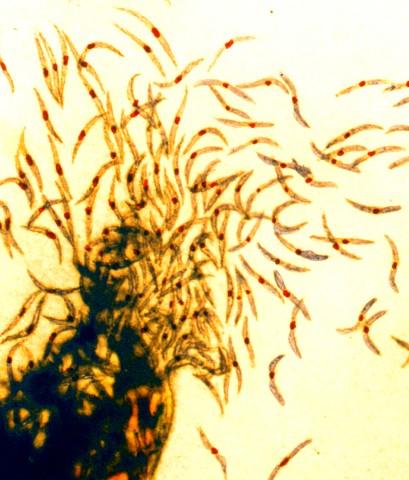
Malaria sporozoites, the infectious form of the malaria parasite that a mosquito’s bite transmits to humans.
Finally, but perhaps most importantly, the modified mosquitos were much less likely to transmit malaria from an infected mouse to a non-infected mouse compared to their genetically normal counterparts. This result, in particular, provides strong evidence that the IRP team’s genetically modified mosquitos are much less likely to spread malaria than their counterparts in the wild.
Of course, genetically modified mosquitos need to be released into areas where malaria is common and largely replace the wild mosquito population there in order to curb the illness’ transmission. This will require not only additional experiments to verify that releasing the mosquitos would be safe, but also continued work on ‘gene drives’ that would help the mosquitos’ genetic alterations spread through local mosquito populations. Once these obstacles are overcome, though, genetically modified mosquitos like the ones created in Dr. Vega-Rodriguez’s lab could one day help save thousands of lives.
“Malaria is an extremely complex disease, and until we get a silver bullet like a vaccine that is highly effective, we have to target this disease from many angles — not only transgenic mosquitos, but also insecticides, drugs, and any other intervention that could prevent the transmission of the disease,” he says. “It could be my transgenics, it could be another group’s, but if we are able to replace the mosquito population with mosquitos that are highly resistant to the parasite, it would certainly have significant impact in reducing the transmission of this deadly disease.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Pascini TV, Jeong YJ, Huang W, Pala ZR, Sá JM, Wells MB, Kizito C, Sweeney B, Alves E Silva TL, Andrew DJ, Jacobs-Lorena M, Vega-Rodríguez J. Transgenic Anopheles mosquitoes expressing human PAI-1 impair malaria transmission. Nat Commun. 2022 May 26;13(1):2949. doi: 10.1038/s41467-022-30606-y.
[2] Alves E Silva TL, Radtke A, Balaban A, Pascini TV, Pala ZR, Roth A, Alvarenga PH, Jeong YJ, Olivas J, Ghosh AK, Bui H, Pybus BS, Sinnis P, Jacobs-Lorena M, Vega-Rodríguez J. The fibrinolytic system enables the onset of Plasmodium infection in the mosquito vector and the mammalian host. Sci Adv. 2021 Feb 5;7(6):eabe3362. doi: 10.1126/sciadv.abe3362.
Related Blog Posts
This page was last updated on Tuesday, May 23, 2023
