Newly Discovered Mutation Causes Eye Disease
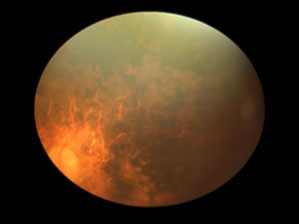
This image shows the damaged retina of a patient with a genetic mutation discovered by IRP investigators that causes the eye disease retinitis pigmentosa.
The Human Genome Project gave scientists an incredible roadmap of the thousands of genes used to construct the human body. However, many individuals harbor DNA that differs markedly from the standard reference sequence produced by that initiative, and these variations can have profound implications for a person’s health. A recent study led by IRP scientists has uncovered yet another of these genetic variants, a rare mutation that causes the eye disease retinitis pigmentosa.1
Retinitis pigmentosa is one of the most common diseases of the retina, the part of the eye that contains light-sensing cells called photoreceptors. In patients with the condition, the photoreceptors degenerate over time, leading first to poor night vision and deteriorating peripheral vision and eventually causing substantial vision loss that leaves patients legally blind.
Mutations in more than 50 genes are known to cause retinitis pigmentosa. IRP senior investigator J. Fielding Hejtmancik, M.D., Ph.D., has been working for over a decade with researchers at Johns Hopkins University and the Center for Excellence in Molecular Biology in Lahore, Pakistan, to identify previously unknown genetic mutations that cause inherited eye diseases. By sequencing the DNA of 143 Pakistani families containing several members with retinitis pigmentosa, the project discovered that multiple affected individuals in five families had a never-before-seen change in a gene called CLCC1, which provides the genetic blueprint for building a channel that moves around chloride ions within cells. Around the same time, a research group in the UK found the same mutation in three other families with Pakistani ancestry and a family history of retinitis pigmentosa, which spurred a collaborative effort to investigate it.
“Finding that this mutation in an intracellular chloride channel caused the disorder threw us for a bit of a loop,” Dr. Hejtmancik says. “It’s not part of the other groups of proteins known to be involved in the disease. Finding something that’s completely outside those groups was a bit of a surprise for us.”
Prior to that discovery, the CLCC1 chloride channel had been largely ignored by the scientific community, with just a single study having examined it nearly three decades ago.2 Yet the gene’s sequence is remarkably similar across a number of different species from zebrafish to mice to humans, suggesting that it plays an important role in cells.
When Dr. Hejtmancik’s team inserted a mutated version of the CLCC1 channel into human and chicken retinal cells, the abnormal molecule accumulated in a structure called the endoplasmic reticulum that generates and transports cellular proteins. In addition, when Dr. Hejtmancik’s team knocked down the activity of the gene in lab-grown human retinal cells, roughly 10 percent of the cells activated a cellular self-destruct process and died, compared to less than one percent of control cells.
“It’s sort of a double whammy,” Dr. Hejtmancik says. “The absence of CLCC1’s function will kill the cell, and having that damaged protein hanging around in the endoplasmic reticulum probably doesn’t help either.”
Further experiments showed that zebrafish larvae without the CLCC1 gene had abnormal retinas with fewer photoreceptors, which showed signs of degeneration. Injecting these zebrafish with genetic material that allowed their cells to manufacture the CLCC1 channel partially reversed those abnormalities. Mice missing just one copy of the gene had similar retinal defects.
More work will be needed to pin down precisely what the CLCC1 chloride channel does in cells both within and outside the retina, as well as why the CLCC1 mutation causes retinitis pigmentosa. Even without that knowledge, Dr. Hejtmancik’s findings will enable genetic counseling for retinitis pigmentosa patients with the CLCC1 mutation, and further down the line it may be possible to correct the mutation’s consequences using gene therapy.
“We might clinically do some good for some patients at some point, especially if we can do gene therapy,” Dr. Hejtmancik says. “But in the near term, this study really serves as a guidepost for future investigations into the physiology and biochemistry of the retina. It provides a foundation for all the other studies that will be done, many of which will have practical implications.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Mutation in the intracellular chloride channel CLCC1 associated with autosomal recessive retinitis pigmentosa. Li L, Jiao X, D'Atri I, Ono F, Nelson R, Chan CC, Nakaya N, Ma Z, Ma Y, Cai X, Zhang L, Lin S, Hameed A, Chioza BA, Hardy H, Arno G, Hull S, Khan MI, Fasham J, Harlalka GV, Michaelides M, Moore AT, Coban Akdemir ZH, Jhangiani S, Lupski JR, Cremers FPM, Qamar R, Salman A, Chilton J, Self J, Ayyagari R, Kabir F, Naeem MA, Ali M, Akram J, Sieving PA, Riazuddin S, Baple EL, Riazuddin SA, Crosby AH, Hejtmancik JF. PLoS Genet. 2018 Aug 29;14(8):e1007504. doi: 10.1371/journal.pgen.1007504. [Epub ahead of print]
[2] Identification of a novel chloride channel expressed in the endoplasmic reticulum, golgi apparatus, and nucleus. Nagasawa M, Kanzaki M, Iino Y, Morishita Y, Kojima I. J Biol Chem. 2001 Jun 8;276(23):20413-8. Epub 2001 Mar 5.
Related Blog Posts
This page was last updated on Tuesday, January 30, 2024
