Research Briefs
NIBIB, NHLBI, NICHD, NCI: ARTIFICIAL INTELLIGENCE AND HARDWARE INNOVATIONS ENHANCE MICROSCOPY
CREDIT: NIH MEDICAL ARTS
Artist rendition of the triple-view line scanning confocal microscope obtaining an image of the roundworm, C. elegans, in conjunction with deep learning.
Confocal microscopy is a powerful tool in biomedical research. By using a focused beam of laser light, narrow slices of a biological sample are scanned and then digitally reconstructed to create 3D images. However, the technology has limitations: Images become blurry along the third dimension, and resolution diminishes in thicker samples. An NIBIB-led team of researchers and their collaborators recently developed an improved confocal microscope capable of generating higher-quality images. The scientists captured remarkable images of more than 20 biological structures and processes ranging from nanoscale dynamics of proteins within immune cells to developing nerve cells in roundworm embryos. Their findings were published in Nature.
Using a technique called triple-view line-scanning confocal microscopy, the researchers sequentially scanned slices of a sample from three directions with the new microscope. Computer software then stitched together the separate images into a single super-resolution 3D image. The investigators then applied a form of artificial intelligence called deep learning to process images scanned at low light levels. Lower light is sometimes used to avoid damaging biological structures and allow longer imaging times, but can result in poor resolution. Deep-learning algorithms were able to compensate for the blurry images and produce a higher-quality image than the original.
From the molecular to the tissue level, the authors hope these new approaches will be used to deepen our understanding of how organisms develop and are organized. (NIH authors: Y. Wu, X. Han, Y. Su, J. Liu, R. Fischer, C. Combs, J. Sun, X. Wu, R. Christensen, L. Bao, Y. Sun, J. Chen, Y. Pommier, Y. Shi, E. Murphy, and H. Shroff, Nature 600:279–284, 2021; DOI:10.1038/s41586-021-04110-0)
NICHD, NHGRI, NIAMS: ALS DRUG MAY HELP TREAT RARE NEURODEGENERATIVE DISORDER
In a mouse model, NIH scientists have repurposed riluzole, a drug currently approved to treat amyotrophic lateral sclerosis (ALS), as a potential treatment for Niemann-Pick disease type C1 (NPC1). NPC1 is a rare genetic disorder typically affecting children and adolescents. The gradual loss of brain cells known as Purkinje neurons in the cerebellum is a hallmark of the disease, resulting in difficulty coordinating movements, cognitive decline, and death.
An NICHD-led team first analyzed RNA sequencing data from mice with a form of NPC1 and observed decreased expression of a gene that encodes a protein that transports glutamate (an excitatory neurotransmitter) in the brain. High concentrations of glutamate can be toxic to neurons, which the researchers hypothesized was playing a role in NPC1 disease progression.
The investigators then treated the NPC1 mice with the FDA-approved drug riluzole, which reduces the release of glutamate, and found that treated mice survived 12% longer than untreated ones. The findings suggest that riluzole or similar glutamate-inhibiting drugs could be used as a new therapeutic to treat patients with NPC1. (NIH authors: A. Cougnoux, J.C. Yerger, M. Fellmeth, J. Serra-Vinardell, F. Navid, C.A. Wassif, N.X. Cawley, and F.D. Porter, Mol Genet Metab 134:330–336, 2021; DOI:10.1016/j.ymgme.2021.11.008)
NIA, NCI: CANCER DRUGS BEING TESTED TO FIGHT ALZHEIMER DISEASE
CREDIT: ATSUSHI SAITO, JOHNS HOPKINS UNIVERSITY SCHOOL OF MEDICINE
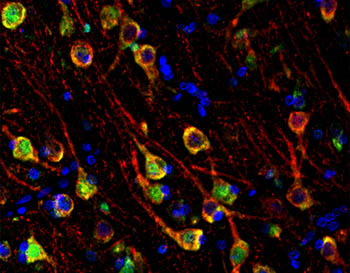
The image shows that LGALS8, one of the proteins altered in young APOE4 carriers as well as in brains with Alzheimer disease, is located inside neurons in the brain. LGALS8 proteins are stained green and neurons are stained red. Cells that are stained yellow are those where both green and red dyes are in the same location.
NIA researchers and their collaborators have discovered that young individuals who are genetically susceptible to Alzheimer disease (AD) may benefit from drugs currently used to treat cancer.
In a recent study, scientists analyzed post mortem brain samples from participants of two existing studies who carried APOE4, a known AD genetic-risk variant. The researchers identified protein changes associated with APOE4 in the brains of participants with an average age at death of 39 years and compared them with the brains of those who died at an average age of 89 years, with and without AD. APOE4-associated changes observed in mouse models further confirmed their findings.
Using in vitro experiments, the investigators then tested whether existing FDA-approved or experimental drugs targeted the identified proteins, such as signal transducer and activator of transcription 3, YES1, and FYN. Indeed, the team found that an experimental drug for liver cancer, and dasatinib (used to treat chronic myeloid leukemia), acted upon the AD-related proteins. In the ongoing Drug Repurposing for Effective Alzheimer’s Medicines study, NIA scientists are analyzing large real-world clinical datasets to test whether commonly used drugs like dasatinib may be effective Alzheimer treatments. [https://www.youtube.com/watch?v=VzAueww60GE&feature=youtu.be.] (NIH authors: J. Roberts, V.R. Varma, Y. An, J. Candia, G. Fantoni, V. Tiwari, C. Anerillas, A. Williamson, R. Moaddel, M. Khadeer, J. Lovett, T. Tanaka, D.L. Croteau, S.M. Resnick, M. Gorospe, V.A. Bohr, L. Ferrucci, and M. Thambisetty, Sci Adv 7:eabi8178, 2021; DOI:10.1126/sciadv.abi8178)
NIAID: HIV-INFECTED INDIVIDUALS CONTROL THE VIRUS IN DIFFERENT WAYS
CREDIT: NIAID
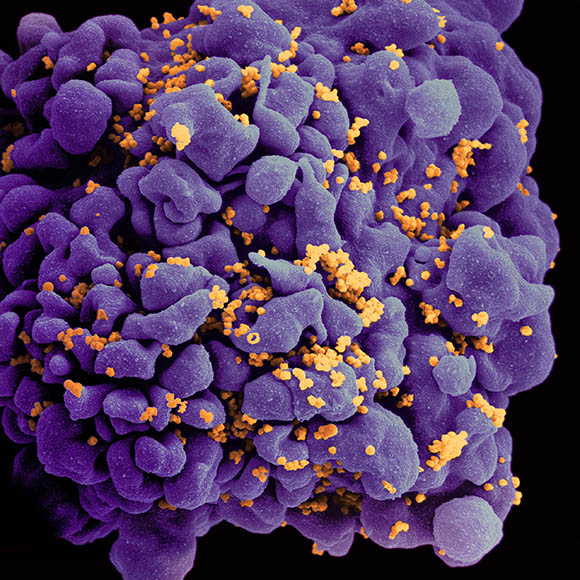
NIAID: Scanning electron micrograph of an HIV-infected (orange) H9 T cell.
NIAID scientists have identified two distinct ways in which HIV-infected individuals were able to control the virus after stopping antiretroviral therapy (ART). ART is effective at suppressing HIV, but is a lifelong medication regimen that can have long-term side effects and the potential for developing drug resistance.
In this study, two adult participants began ART soon after acquiring HIV and continued treatment for over six years, successfully suppressing the virus. They then joined an HIV clinical trial and stopped taking ART under medical supervision. The participants were monitored for viral rebounds (times when the amount of HIV in their blood became detectable) every two to three weeks over the course of several years.
The first participant suppressed the virus with intermittent rebounds for nearly 3.5 years until drug testing revealed that he had begun taking undisclosed, suboptimal ART. The investigators found that this participant (but not the second one) had high concentrations of HIV-specific immune cells called CD8+ T cells that may have contributed to his control of the virus.
For nearly four years, the second participant almost completely suppressed HIV and showed a very strong neutralizing antibody response not seen in the first participant. The authors suggest that this near-complete HIV suppression may have been facilitated by the neutralizing antibodies. This individual eventually experienced a dramatic viral rebound when he became infected with a different HIV strain, a phenomenon known as “superinfection.”
Understanding the mechanisms of virus-suppression in these two individuals could provide insight into the development and application of new treatments for HIV. (NIH authors: J. Blazkova, J.S. Justement, V. Shi, E.J. Whitehead, R.F. Schneck, E.D. Huiting, K. Gittens, J. Lack, M.C. Sneller, S. Moir, A.S. Fauci, and T.W. Chun, Nat Med 27:1893–1898, 2021; DOI:10.1038/s41591-021-01503-6)
NICHD: EARLY CHILDHOOD EATING PROBLEMS LINKED TO DEVELOPMENTAL DELAY
In a recent study, NICHD researchers found that frequent eating problems in the first three years of life could be used to identify children at risk for developmental delay.
Scientists analyzed data from 3,597 children born between 2008 and 2010 in New York State whose mothers reported their children’s feeding behavior and developmental progress as part of Upstate KIDS, a population-based birth cohort study. When the children were 18, 24, and 30 months old, each mother rated their child’s eating problems in several categories including food refusal, mechanical (swallowing) issues, and distress such as crying during meals. Using the Ages and Stages Questionnaire, developmental milestones were assessed at the same three time points.
The results revealed that children with feeding problems were more likely to be scored as developmentally delayed. This was especially true as the frequency of eating problems increased. Compared with kids with no trouble eating, children with frequent feeding problems at one or two time points were more than twice as likely to miss developmental milestones, and those with frequent feeding problems at three time points were more than four times as likely to show developmental delay.
The authors noted that neurological, motor, or communication deficits associated with developmental delay may underlie feeding issues, and that young children who frequently have difficulty eating may benefit from targeted screening. (NIH authors: D.L. Putnick, S.L. Robinson, R. Sundaram, and E. Yeung, J Pediatr 2021; DOI:10.1016/j.jpeds.2021.11.010)
NIEHS: AUGMENTING IMMUNE RESPONSE MAY EFFECTIVELY TREAT PNEUMONIA
REDIT: HONG LI, NIEHS
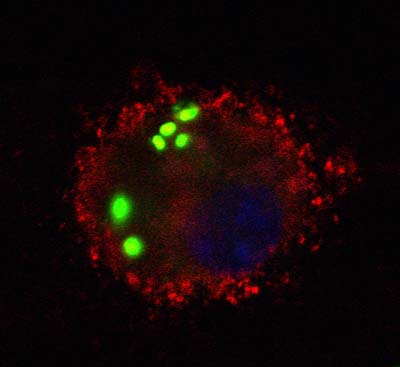
S. pneumoniae bacteria (green) that have been engulfed by a macrophage from a wild-type mouse.
Pneumonia, commonly caused by Streptococcus pneumoniae bacteria, is a leading cause of mortality in the United States. Treatment can be limited by antibiotic drug resistance, and a recent study led by NIEHS scientists has shown how targeting the body’s own immune response may provide an alternative treatment option.
During lung infection caused by bacteria, a natural inflammatory response prompts white blood cells called macrophages to consume and remove the pathogens. To keep tissues healthy and control inflammation, signaling compounds known as epoxyeicosatrienoic acids (EETs) are also produced, but can inhibit macrophage activity.
The research team discovered that infection induced a protein called soluble epoxide hydrolase (sEH), which degraded EET and allowed macrophages to more effectively clear bacteria. When sEH was blocked in genetically modified mice, EET concentrations spiked, hampering the macrophages’ ability to eat bacteria and resulting in more severe lung infections. In contrast, when EET was blocked using a synthetic molecule called EEZE, the scientists observed an increase in the eating capacity of the macrophages, leading to reduced numbers of bacteria in the lungs of mice. Identical results were seen when the authors studied the interaction between bacteria and human lung macrophages in vitro.
“EEZE is safe and effective in mice, but scientists could develop similar compounds to give to humans,” said Matthew Edin, co–lead author of the paper. (NIH authors: H. Li, J.A. Bradbury, M.L. Edin, J.P. Graves, A. Gruzdev, J. Cheng, S.L. Hoopes, L.M. DeGraff, M.B. Fessler, S. Garantziotis, S.H. Schurman, and D.C. Zeldin, J Clin Invest 131: e129679, 2021; DOI:10.1172/JCI129679)
[BY SATABDI NANDI, NIA]
This page was last updated on Friday, January 28, 2022
