COVID-19 Timeline at NIH (November–December 2021)
COVID-19 Research and Activities at NIH (November–December 2021)
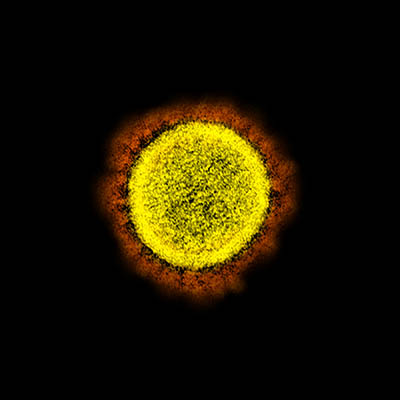
CREDIT: NIAID
Transmission electron micrograph of SARS-CoV-2 virus particles, isolated from a patient. Image captured and color-enhanced at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland.
November 2: NICHD supports a four-year follow-up study on the potential long-term effects of COVID-19 on women infected with SARS-CoV-2 during pregnancy.
November 3: NIH’s Rapid Acceleration of Diagnostics initiative announces the launch of the When To Test Calculator for Individuals. By answering a few prompts, the online tool helps people determine whether they are at risk of getting or transmitting COVID-19 and make informed testing decisions.
November 4: The Department of Health and Human Services (HHS) announces its plan for a phased return to the physical workplace, replacing the NIH framework in place since June 2020. NIH Director Francis Collins follows up with an email to all staff that the NIH return to the physical workplace intranet page has been updated to reflect this new plan, and that he expects workplace flexibilities to continue for most staff.
November 4: An NIH commentary published in Pediatrics highlights the need for research that involves community engagement in underserved or vulnerable areas of the United States for safe in-person school during the COVID-19 pandemic. (Pediatrics e2021054268B, 2021; DOI:10.1542/peds.2021-054268B)
November 4: HHS Secretary Xavier Becerra and Deputy Secretary Andrea Palm visit NIH to meet with top NIH leadership. The HHS colleagues get a tour of a Clinical Center lab and a lab demonstration at the Vaccine Research Center.
November 5: NIDCR researchers identify an enzyme process that may influence infectivity of SARS-CoV-2 variants and potentially boost the virus’s ability to spread. (PNAS 118:e2109905118, 2021; DOI:10.1073/pnas.2109905118)
November 9: The New York Times (NYT) reports that Moderna seeks to exclude U.S. government scientists from its COVID-19 vaccine patents. The vaccine was the result of a collaboration with NIH’s Vaccine Research Center researchers who worked with Moderna to design the genetic sequence that prompts the mRNA vaccine to produce an immune response. Results from this collaboration played “a major role in the development of the vaccine,” according to NIH Director Francis Collins who was quoted in a Nature Biotechnology article published in December. “It’s not a good idea to file a patent when you leave out important inventors, and so this is going to get sorted [out] as people look harder at this.” (Nat Biotechnol 39:1481, 2021)
November 9: A study featured in the “NIH Director’s Blog” shows waning COVID-19 immunity over time after vaccination. (N Engl J Med 385:e85, 2021; DOI:10.1056/NEJMoa2114228)
November 12: In his weekly email, NIH Director Francis Collins reminds federal employees to report their vaccination status by the November 22 deadline, and that the deadline for contractors has been extended to January 18, 2022. He reports that asymptomatic testing will be offered on Mondays and Tuesdays through the end of the year.
November 15: HHS withdraws a policy established during the previous administration that limited FDA’s ability to assess whether certain COVID-19 tests work as intended, ensuring that tests are accurate, reliable, and available.
November 15: NIAID launches a three-year study that will evaluate the physical and mental health of up to 1,000 children and young adults who previously tested positive for COVID-19.
November 16: On his blog, NIH Director Francis Collins features early clinical trial data from Pfizer that show that their new orally administered therapeutic drug may prevent severe illness and hospitalization in people with COVID-19. (Science 374:1586–1593, 2021; DOI:10.1126/science.abl4784)
November 17: NIH scientists and their collaborators publish a study that shows how SARS-CoV-2 spreads in the lungs and prevents tissue repair, revealing trends that could help develop new COVID-19 treatments and fine-tune when to use existing therapeutics. (Sci Transl Med 13:Issue 620, 2021; DOI:10.1126/scitranslmed.abj7790)
November 18: The “NIH Director’s Blog” covers an NIH-funded study demonstrating how the SARS-CoV-2 delta variant packs more of its genetic material into an infected host cell and may help explain delta’s increased transmissibility. (Science 374:1626–1632, 2021; DOI:10.1126/science.abl6184)
November 24: HHS Secretary Xavier Becerra announces to staff that more than 96% of HHS employees have been vaccinated against COVID-19.
November 24: A new variant of SARS-CoV-2, B.1.1.529 (omicron), was reported to the World Health Organization.
November 28: Speaking to CNN about the newly detected omicron variant, NIH Director Francis Collins says, “We don’t know yet how much of an impact this will have. It ought to redouble our efforts to use the tools that we have, which are vaccinations and boosters…It also means we need to pay attention to those mitigation strategies that people are just really sick of, like wearing masks while indoors with other people who might not be vaccinated and keeping that social distance issue.”
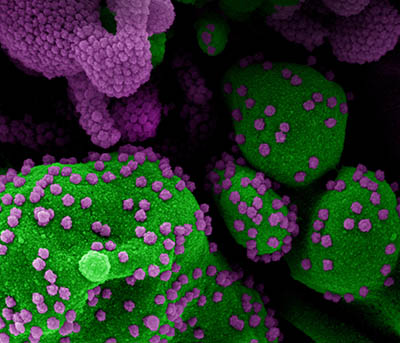
CREDIT: NIAID
Colorized scanning electron micrograph of an apoptotic cell (green) heavily infected with SARS-COV-2 virus particles (purple), isolated from a patient sample. Image from the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland.
December 1: The first case of COVID-19 attributed to omicron was reported in the United States.
December 2: President Joseph Biden visits NIH for a live broadcast event and speaks about the White House’s strategy for fighting COVID-19 this winter.
December 2: The Centers for Disease Control and Prevention (CDC) announces that it is revising the current Global Testing Order to shorten the timeline for required testing for all international air travelers to one day before departure to the United States. This revision strengthens already robust protocols in place for international travel, including requirements for foreign travelers to be fully vaccinated.
December 6: HHS Secretary Xavier Beccera reports that Phase 2A employees in leadership positions and those supporting them will begin returning to the workplace this week. However, plans to return the approximately 11,000 employees in Phase 2B and 2C have been paused. NIH Director Francis Collins follows up on December 7 with an email to staff that the NIH Return to the Physical Workplace intranet page will be updated as more information becomes available.
December 10: Francis Collins sends his last biweekly Friday coronavirus update email to staff as NIH director before stepping down on December 19 and remarks on the privilege of hosting President Joseph Biden on campus December 2. He reports on the importance of boosters now that new SARS-CoV-2 infections in the United States are on the upswing and that the new omicron variant has been detected in more than 20 states. Collins concludes by expressing gratitude for the staff who keep NIH running.
December 13: The Gateway Vehicle Inspection Station (Building 66A) off Rockville Pike re-opens on weekdays only to help alleviate morning congestion at the Commercial Vehicle Inspection Facility. The partial reopening of Building 66A is due in part to the need to continue to operate a COVID-19 testing site for symptomatic or for-cause staff at this location. Visitor screening and testing operations will not overlap.
December 14: On his “NIH Director’s Blog,” Francis Collins writes, “There has been great concern about the omicron variant of SARS-CoV-2….The main reason is that it has accumulated over 50 mutations, including about 30 in the spike protein….All of these genetic changes raise the possibility that omicron could cause breakthrough infections in people who’ve already received a Pfizer or Moderna mRNA vaccine.”
December 15: The latest results from the NIDA-supported Monitoring the Future study show that the percentage of adolescents reporting substance use decreased significantly in 2021 as the COVID-19 pandemic went on. These findings represent the largest one-year decrease in overall illicit drug use reported since the survey began in 1975.
December 16: NIAID Director Anthony Fauci and NIAID scientists Jeffery Taubenberger and David Morens publish a commentary in The New England Journal of Medicine urging the global scientific and medical research community to pursue a universal coronavirus vaccine to counter future outbreaks. (N Engl J Med 2021; DOI:10.1056/NEJMp2118468)
December 16: NIH hosts a virtual farewell tribute to NIH Director Francis Collins who will be stepping down as director on December 19.
December 17: The Office of Research Services (ORS) reports that they have successfully vaccinated 16,082 staff at NIH and given boosters to over 3,434 employees this past year. ORS has administered all their allocated and available doses and will no longer be scheduling appointments. Incoming staff are required to be vaccinated prior to their start date and will need to be vaccinated in the community.
December 17: The New York Times reports that Moderna has backed down from its dispute with NIH over who deserves credit for a crucial component of its coronavirus vaccine.
December 19: NIH Director Francis Collins steps down as director and will resume working in his NHGRI lab fulltime.
December 20: NIH Principal Deputy Director Lawrence A. Tabak begins serving as the acting director of NIH.
December 22: HHS announces new guidance to boost accessibility and equity in COVID-19 vaccine programs to ensure nondiscrimination on the basis of race, color, and national origin.
December 27: CDC announces that it has updated and shortened the recommended isolation period (for people who test positive for COVID-19) and quarantine period (for people exposed to someone with COVID-19).
December 28: The Clinical Center announces that beginning January 3, 2022, the asymptomatic COVID-19 testing clinic will expand its schedule from three days a week to four (Monday-Thursday). Both saliva and mid-turbinate swab testing are offered. Appointments are required (https://clinweb.cc.nih.gov/cct; access to website limited to NIH VPN, NIH IC CITRIX, or from NIH workstations).
December 29: HHS announces that the Biden-Harris Administration has brought two new over-the-counter, at-home COVID-19 tests to the U.S. market. The tests, one manufactured by SD Biosensor and distributed by Roche and the other manufactured by Siemens, have received emergency use authorization (EUA) by the FDA. These quick authorizations are thanks to collaboration between the FDA and the NIH Rapid Acceleration of Diagnostics Technology (RADx) program. Combined, it is estimated the companies can produce tens of millions of tests per month for use in the U.S.
This page was last updated on Friday, January 28, 2022
