Toxic Protein and Aging Combine Forces to Drive Brain Disease
NIA Study Suggests New Therapeutic Targets for Pair of Age-Related Illnesses
Aging wears down all parts of our bodies, from our bones to our brains. It’s no surprise, then, that it’s the main risk factor for neurological illnesses like Parkinson’s disease and dementia. However, the precise reason why has long remained a mystery. New research from the National Institute on Aging (NIA) suggests that the aged brain is a fertile ground for the spread of a harmful protein associated with several neurological diseases, and that the toxic protein itself ages immune cells in the brain (Mol Neurodegener 17:60, 2022).
CREDIT: NIA
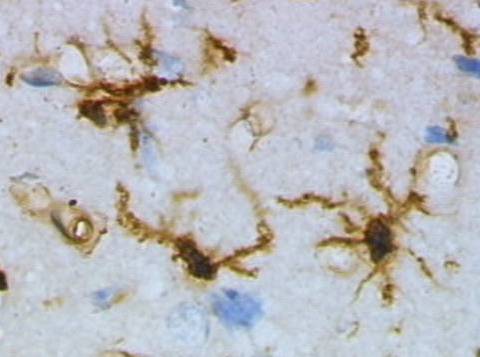
An NIA study suggested that alpha-synuclein drives aging and makes microglia in younger mice act more like those in older ones. Shown: Microglia stained with a brown chemical and viewed under a microscope.
Just as research on Alzheimer’s disease has focused primarily on two proteins called amyloid-beta and tau, scientists have honed in on a protein called alpha-synuclein as the probable main villain responsible for Parkinson’s disease, in which the death of certain neurons in the brain causes cognitive and movement problems. Like amyloid-beta and tau in Alzheimer’s disease, alpha-synuclein builds up in the brains of patients with Parkinson’s disease and a form of dementia called Lewy body dementia (LBD).
NIA Senior Investigator Eliezer Masliah has spent his career studying alpha-synuclein and its role in neurological illnesses. However, until he came to NIA, his experiments tended not to take into account the influence of aging on those diseases.

CREDIT: NIA
Eliezer Masliah
“I spent 30 years in academia doing this work and only occasionally thought about aging,” said Masliah, the new study’s co-senior author along with NIA Senior Investigator Ranjan Sen. “We were studying all these models of these diseases and testing all these drugs and we were using mostly middle-aged animals. Really, it wasn’t until I came to NIA and started to hear so much about aging from my colleagues and heard about all the new mechanisms people were discovering behind aging that I became more interested in it.”
With this new perspective in mind, Masliah’s lab teamed up with Sen’s group in order to determine how aging interacts with alpha-synuclein in the brains of mice. To do so, the researchers injected alpha-synuclein protofibrils into the brains of two sets of mice: one group that was only three to four months old and another that was 18 to 19 months old. These protofibrils can induce similar consequences in mice to those seen in humans with Parkinson’s or LBD because they cause alpha-synuclein to build up in, and spread through, the animals’ brains.

CREDIT: NIA
Ranjan Sen
In fact, the study found, the alpha-synuclein spread much more in the brains of the older mice than in those of the younger animals. What’s more, the older mice that received alpha-synuclein injections showed less of a fear response to a sound that they had previously been taught to associate with a mild foot shock, suggesting their memories were impaired. In the younger mice, on the other hand, the alpha-synuclein injections had much less of an effect on their response to the fear-inducing sound. Interestingly, while the NIA scientists observed alpha-synuclein’s effects on cognition in both male and female mice, the protein only appeared to decrease motor coordination in male mice.
“There has been a lot of interest at NIA about understanding sex differences in neurodegenerative disorders,” Masliah said. “We know in Lewy body dementia that women are affected differently than men, and also Alzheimer’s disease tends to be more common in women than in men. There may be a difference in how sex hormones affect the disease or some other factor that we don’t understand, but that’s why it’s important when designing these sorts of experiments to include both sexes.”
The alpha-synuclein injections also appeared to influence the number and behavior of immune cells in the animals’ brains. Mice that received them had more immune cells called T cells and microglia in their brains one month later compared to untreated mice, and these cells were more active as well. Furthermore, these effects were amplified in the older mice compared to the younger mice.
When the researchers examined the activity of genes in those microglia, they found that the alpha-synuclein injections altered the activity of numerous genes related to inflammation. Many of those genes also behaved differently in older mice compared to younger mice even when the animals did not receive alpha-synuclein injections, suggesting that alpha-synuclein makes microglia in younger mice act more like those in older mice. In addition, when the researchers conducted an analysis to determine how those genes are regulated, they found that some of the genes are influenced by a chemical called colony stimulating factor 2 (CSF2), which is secreted by T cells and certain other immune cells. That finding bolsters those of other research that has only recently begun to link CSF2 to neurological illnesses.
“I think the fact that we found a link to CSF2 suggests that all these excess T cells that are getting into the brain might be secreting more CSF2, and this CSF2 is detected by microglia,” Masliah explained. “The microglia become activated, and then we see dysregulation in all these other inflammatory pathways. That’s very important because it suggests that targeting either CSF2 or its receptor in microglia might prevent the acceleration of aging in microglia that appears to be triggered by alpha-synuclein.”
Now that the study has given Masliah’s and Sen’s teams a broad view of how alpha-synuclein and aging affect the brains of mice, the group plans to study the effects of those factors in mice that lack specific types of immune cells in their brains, such as T cells or microglia. They are also curious to investigate how alpha-synuclein and aging affect the brains of mice with immune cells that either can’t produce or can’t respond to CSF2. Such experiments will build on the findings of the recent study to produce a more detailed picture of the ways both aging and alpha-synuclein wreak havoc on the brain, information that is critical for creating new treatments.
“Our findings suggest that pro-inflammatory pathways that are triggered by aging could also be triggered in a similar way by accumulation of alpha-synuclein,” said Masliah. “They are running in parallel and also probably feeding into each other. I think we can develop therapeutic strategies for diseases like Parkinson’s and Lewy body dementia by looking at these age-related inflammatory pathways.”

Brandon Levy is a Health Communications Specialist for NIH’s Intramural Research Program. When he’s not hunched over a computer keyboard, he enjoys singing in his acapella group, reading, honing his skills as an amateur chef, and over-obsessing about college basketball.
This article is adapted from the December 20, 2022, post in the I Am Intramural Blog: https://irp.nih.gov/blog/post/2022/12/toxic-protein-and-aging-combine-forces-to-drive-brain-disease.
This page was last updated on Monday, January 9, 2023
