Searching for Answers
NIH’s Undiagnosed Diseases Program Grows Into a Worldwide Model
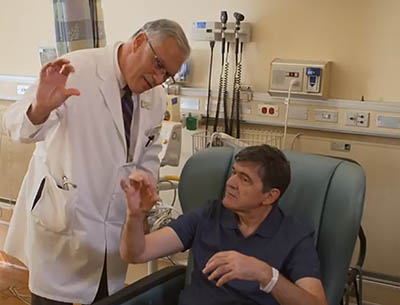
Credit: From “Zarko’s Story: Genomic Medicine In Action”
None of Zarko Stanacev’s physicians could figure out what was causing attacks that rendered him nearly comatose, confined him to a wheelchair, and cloaked him in profound fatigue. NIH’S Undiagnosed Diseases Program, however, found a mutation in the NLRP3 gene that was causing abnormal inflammation in his body. Hours after being given a drug to treat inflammation, he dramatically improved and was up and walking without pain. Pictured: Zarko Stanacev (sitting) and NHGRI neurologist Camilo Toro (standing) who led the team that treated Stanacev. (Credit: From “Zarko’s Story: Genomic Medicine In Action” at https://www.youtube.com/watch?v=McVwgeHa1Fk)
Zarko Stanacev had suffered debilitating symptoms for more than a decade. What started out as episodes of hearing loss and severe headaches escalated to periodic seizures and meningitis. The attacks rendered him nearly comatose, confined him to a wheelchair at times, and cloaked him in profound fatigue. His medical providers were mystified. Without answers, the prospect of his living a normal life seemed out of reach.
But in 2017, Stanacev was accepted into NIH’s Undiagnosed Diseases Program (UDP), where an interdisciplinary team led by National Human Genome Research Institute (NHGRI) neurologist Camilo Toro set out to discover the source of his puzzling symptoms. They found a mutation in the NLRP3 gene that was causing abnormal inflammation in Stanacev’s body. Hours after being treated with anakinra, a drug used to treat inflammation, he dramatically improved and was up and walking without pain. Finally, he had hope and a path back to a normal life.
For many patients, the UDP represents their last, best hope. A component of the Intramural Research Program, the UDP is a trans-NIH initiative hosted by NHGRI. UDP Director William Gahl started the program in 2008 with $280,000 of seed funding from the Office of Rare Diseases Research (ORDR).

CREDIT: MAGGIE BARTLETT, NHGRI
In 2008, William Gahl established the NIH Undiagnosed Diseases Program. Ever since it has been finding answers to the most puzzling medical cases from around the world.
“Steve Groft, director of the ORDR at the time, was getting calls from patients whose diseases had no name. They had no diagnosis,” said Gahl. “Clearly, there was an unmet need.” And the UDP could meet that need.
In the beginning, the UDP had only a staff of four: Gahl, a scheduler, and two nurse practitioners. “I expected we would answer a few people’s questions, do some research in my lab on new diseases, perhaps discover new pathways which might apply to treatments,” he said. Then, two things happened that propelled the UDP beyond Gahl’s expectations.
First, in May 2008, then–NIH Director Elias Zerhouni and other NIH leaders held a press teleconference to announce the UDP. In addition to reporters, representatives from 96 rare-disease organizations were on the phone. “After that, the applications started pouring in,” said Gahl.
Second, next-generation sequencing (NGS) was burgeoning at the time. NGS allowed an entire human genome to be sequenced in a single day instead of years. In addition, whole-exome sequencing (focused on protein-coding regions of DNA) now allowed researchers to selectively search for mutations in a patient’s genetic code that could be causing their symptoms. At the least scientists were able to find answers, and at best, a treatment.
NIH’s laboratories are also certified by the Centers for Medicare and Medicaid Services through the Clinical Laboratory Improvement Amendments. The certification was a costly but necessary distinction for a lab’s results to influence a patient’s care. In 2008, however, there was no CLIA certification anywhere for whole-exome sequencing. Therefore, UDP investigators performed the test on a research basis to detect genetic mutations. Then those results were individually certified to confirm each patient’s variant(s). The UDP had an opportunity to use this technology and do things that were not yet covered by insurance.
Three weeks after the press conference, Gahl sat at his desk staring at an eight-inch stack of medical charts. “I remember thinking, what did I just get myself into?” he said. He leaned on the expertise and generosity of his colleagues across NIH. Since then, the growth of the UDP has been nothing short of remarkable. The program has received more than 4,000 medical records; admitted 1,300 patients (43% were children); sequenced 1,900 exomes and 550 genomes; and made more than 316 diagnoses. And UDP scientists have produced more than 180 publications.
Young researchers have the opportunity to work alongside seasoned investigators to discover new diseases. In 2011, clinical researchers identified the genetic cause of a rare and debilitating vascular disorder associated with progressive and painful calcification of arteries below the waist (New Engl J Med 364:432–442, 2010; DOI:10.1056/NEJMoa0912923). To date, the UDP has discovered 23 new genetic disorders and disease phenotypes. [https://www.genome.gov/news/news-release/bill-gahl-receives-hhs-career-achievement-award]
Today, the UDP’s leadership consists of Camilo Toro, lead neurologist and director of the adult program; Cynthia Tifft, pediatric director; David Adams, attending pediatrician and director of bioinformatics; and May Malicdan, director of translational research. The staff includes internists, neurologists, bioinformatics specialists, and nurse practitioners. All are collaborating with specialists from across NIH.
Medical institutions across the country and all around the world have created programs modeled after the UDP. In 2013, NIH expanded the program to form the Undiagnosed Disease Network, now an extramural network of 12 clinical sites and supporting facilities throughout the United States. By joining the network, the UDP has been able to draw extensive funding from the NIH Common Fund, creating stability and support for the popular program.
Today, nearly half of UDP patients come to NIH with the results of their exome-sequencing in hand. But what the program provides continues to be unique. “We have an unparalleled collection of experts here at NIH that can discuss a case with their colleagues,” Gahl said. “This process would typically take one to two years [but] we can do it in a week.” The treatments are free to the patients. “We can order the tests we think are needed without waiting for insurance to pay.”
The success of the UDP has had a measurable impact. Insurance companies are beginning to cover the cost of genetic sequencing, not only for patients but for their families as well. And, the establishment of UDPs throughout the globe demonstrates how an unmet need is finally being recognized.
Gahl hopes that someday, the UDP will be obsolete. “It’s always been our hope that the program might need to be eliminated,” he said. “If there were no need for a program like this, it would mean that the investigation of rare and new disease had been integrated into our medical-health system.”
The September–October 2010 of the NIH Catalyst includes an article about the UDP’s beginnings. To learn more about the UDP, go to genome.gov/27544402/the-undiagnosed-diseases-program.

Michael Tabasko is the science writer-editor for the NIH Catalyst.
This page was last updated on Monday, February 14, 2022
