COVID-19 Timeline at NIH (March-April 2021)
COVID-19 Research and Activities at NIH
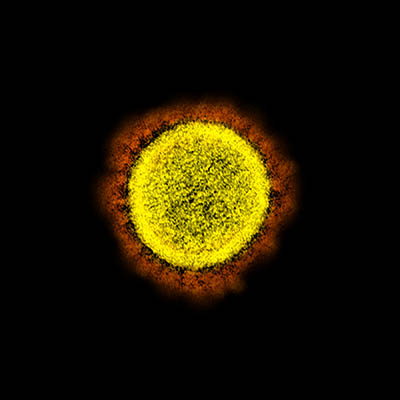
CREDIT: NIAID
Transmission electron micrograph of SARS-CoV-2 virus particles, isolated from a patient. Image captured and color-enhanced at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland.
March 1: An NIH-funded research team launches a study to assess performance and usability of a smartphone app paired with the Quidel QuickVue At-Home COVID-19 Test, which just received an Emergency Use Authorization (link is external) (EUA) from the FDA for use with a prescription.
March 1: NIH halts two Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV)-3 clinical trial substudies for hospitalized patients: one examining the investigational monoclonal antibody therapy VIR-7831, and another evaluating the investigational combination monoclonal antibody therapy containing BRII-196 and BRII-198. There were no safety concerns, but the Data and Safety Monitoring Board (DSMB) determined that there was a low likelihood of clinical value in hospitalized patients.
March 2: NIAID Director Anthony Fauci donates his much-used model of SARS-CoV-2 to the Smithsonian’s National Museum of American History. Fauci is awarded the museum’s signature honor—the Great Americans Medal—for his leadership during the COVID-19 pandemic and lifetime devotion to the treatment and eradication of infectious diseases.
March 2: NICHD announces that it will lead a research effort called “Collaboration to Assess Risk and Identify Long-term Outcomes for Children with COVID (CARING for Children with COVID)”to understand how SARS-CoV-2, the virus that causes COVID-19, affects children, who account for roughly 13% (link is external) of the total cases of COVID-19 in the United States.
March 2: NIH halts a clinical trial evaluating the safety and effectiveness of COVID-19 convalescent plasma in treating emergency department patients who developed mild to moderate symptoms of COVID-19, the disease caused by the coronavirus SARS-CoV-2. NIH determined that the treatment was unlikely to benefit this group of patients.
March 3: NIH Director Francis Collins, in his all-staff email, announces that employees scheduled for qualified urgent medical treatments will be prioritized to receive a COVID-19 vaccine.
March 5: NIH Director Francis Collins updates staff on the improving COVID-19 vaccine supply; NIH received 2,400 Pfizer vaccine second doses from the federal supply chain for Maryland-based employees. Additionally, the Montana Department of Public Health and Human Services supplied 100 Moderna vaccine second doses for staff at NIAID’s Rocky Mountain Labs (Hamilton, Montana). NIEHS in North Carolina also received 230 first doses of the Moderna vaccine. Collins also announces that over the weekend, FDA issued an EUA for the Johnson & Johnson single-dose COVID-19 vaccine.
March 8: NIH launches the last of three clinical trials to evaluate the safety and efficacy of the anticoagulant apixaban 2.5 mg (Eliquis) to prevent life-threatening blood clots. The phase 3 trial is recruiting convalescing adults diagnosed with COVID-19 who are within 45 days of being hospitalized. NHLBI oversees the worldwide trials that are part of the initiative.
March 10: In an all-staff email, NIH Deputy Director for Management Alfred Johnson announces that HHS has extended authorization for supervisors to approve excused absences for employees caring for dependents at home through September 10, 2021.
March 11: The one-year anniversary of the day that the World Health Organization declared the COVID-19 pandemic.
March 12: In his email message to staff, NIH Director Francis Collins acknowledges that the past year has been a long and difficult one. “But there is encouraging news to report: nationally, COVID-19 case numbers are gradually dropping, and with three highly effective COVID-19 vaccines in distribution, more people are being vaccinated every day.” He also reminds staff of the support resources available including about-to-be-launched virtual well-being coaching sessions and a Parents and Caregivers of Children virtual support group.
March 19: NIH Director Francis Collins announces in his all-staff email that all phase 3–eligible employees have been invited by Occupational Medical Services to schedule their COVID-19 vaccination appointments. He notes that vaccine confidence is improving, pointing to an ongoing research project by the Kaiser Family Foundation Vaccine Monitoring Dashboard that tracks the public’s attitudes and experiences with COVID-19 vaccination.
March 22: Results from a large clinical trial, funded in part by NIAID, indicate that AstraZeneca’s COVID-19 vaccine, AZD1222, is well-tolerated and protects against symptomatic COVID-19 disease including severe disease or hospitalization.
March 25: A study led by NIDCR finds evidence that SARS-CoV-2, the virus that causes COVID-19, infects cells in the mouth. The findings suggest that oral cells and saliva play a bigger role in SARS-CoV-2 infection than previously thought, and may contribute to transmission within and outside the body. (Nat Med 2021; DOI:10.1038/s41591-021-01296-8)
March 26: In his all-staff email, NIH Director Francis Collins announces that 2,340 vaccine doses were received to cover all phase 3 employees in Maryland, as well as to phase 4 employees who are 75 and older. In addition, NIEHS in North Carolina received 1,170 first and second doses for staff. Collins also reports that NIH has had its first case of a fully immunized staff member testing positive for SARS-CoV-2 infection more than 14 days after the second dose.
March 30: NIH announces that a NIAID-led team found a largely intact immune response to three of the newly identified variants in recovered COVID-19 patients and vaccinated individuals. The study concludes that the SARS-CoV-2-specific CD8+ T-cells remain active and offer protection against the emerging variants. (Open Forum Infect Dis, 2021; DOI:10.1093/ofid/ofab143)
March 31: The Centers for Disease Control and Prevention (CDC) and NIH launch a community initiative called “Say Yes! COVID Test.” The program will provide free, rapid antigen tests to up to 160,000 residents across two communities located in North Carolina and Tennessee. The effort aims to determine whether frequent, self-administered COVID-19 testing helps residents reduce community transmission of SARS-CoV-2, the virus that causes COVID-19.
March 31: NIAID and biotechnology company Moderna begin phase 1 clinical trials to evaluate the effectiveness of a vaccine designed to protect against the B.1.351 SARS-CoV-2 variant (commonly known as the South African variant).
March 31, 2021: The NIH Community Engagement Alliance (CEAL) Against COVID-19 Disparities holds its first CEAL Alliance Meeting, convening CEAL leadership and members of the 11 CEAL teams to discuss progress, share best practices, and strategize for the future of the Alliance.
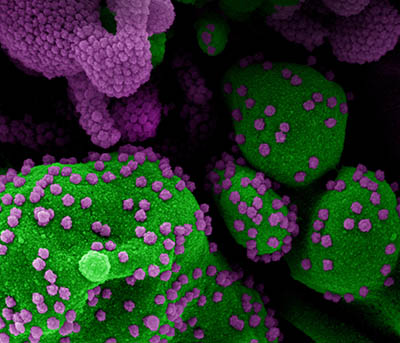
CREDIT: NIAID
Colorized scanning electron micrograph of an apoptotic cell (green) heavily infected with SARS-CoV-2 virus particles (purple), isolated from a patient sample. Image at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland.
April 2: In his email to staff, NIH Director Francis Collins announces that all NIH employees (employees, contractors, and trainees), including teleworkers, are eligible to be vaccinated in their communities under the classification of essential health workers. He reports that CDC announced results of a new agency study providing strong evidence that mRNA COVID-19 vaccines, both Pfizer and Moderna, are highly effective in preventing SARS-CoV-2 infection in real-world conditions among health-care workers, first responders, and other essential workers.
April 5, 2021: CEAL launched the Scientific Pathway, a promotional effort to build confidence in the safety of COVID-19 research by featuring leaders on the pathway from discovery to treatment who share experiences and background with specific communities and what it means to be apart of the Scientific Pathway.
April 7: A new, NIAID-led clinical trial aims to determine whether people who are highly allergic or have a mast cell disorder are at increased risk for an immediate, systemic allergic reaction to the Moderna or Pfizer-BioNTech COVID-19 vaccines.
April 8: NIAID Director Anthony Fauci and colleagues co-author a review article addressing the challenges posed by the ongoing COVID-19 and HIV pandemics. The authors conclude that accelerated development and clinical testing of prevention and treatment strategies are urgently needed to mitigate the dual pandemics. (J Infect Dis 2021; DOI:10.1093/infdis/jiab114)
April 8: In his weekly email message, NIH Director Francis Collins expresses gratitude to all staff by performing a unique take [https://www.youtube.com/watch?v=2QHRPVwa10E] on George Harrison’s “Here Comes the Sun,” made famous by the Beatles.
April 9: Francis Collins announces that NIH has received approval to offer the COVID-19 vaccine to all employees in phase 4 (staff on maximum telework) of the NIH vaccination plan. He also writes that a research letter (authored by NIAID researchers and others) reported interim results of a phase 3 trial of the Moderna COVID-19 vaccine that found antibody activity remained high in all age groups of a small study population (33 healthy adults) at least 6 months after the second dose. (New Engl J Med 2021; DOI:10.1056/NEJMc2103916)
April 9: President Joseph Biden’s budget proposal includes $51 billion for NIH (a $9 billion increase over the 2021 enacted level) to continue to support research that enhances health, lengthens life, and reduces illness and disability.
April 13: The FDA and CDC initiate a pause on the use of Johnson & Johnson COVID-19 vaccine due to an exceedingly rare blood clotting disorder reported in six cases. The pause is lifted on April 23.
April 13: Annals of Internal Medicine publishes a paper describing a workshop in which NIH experts discussed post-acute COVID-19. The NIAID-hosted event drew over 1,200 registered participants including researchers, clinicians, and affected community members, who talked about what is known about sequelae after COVID-19 and the knowledge gaps that need to be addressed in future research. (Ann Intern Med 2021; DOI:10.7326/M21-1043)
April 13: A NIAID-funded clinical trial begins testing an investigational monoclonal antibody for treating patients with COVID-19 who are hospitalized with respiratory disease and low blood oxygen.
April 14: NIH Director Francis Collins serves as a panelist with colleagues from medical institutions across the country during a recent virtual forum titled “Building Vaccine Confidence: Best Practices to Combat Misinformation and Vaccine Hesitancy in COVID-19 Vaccines.” The forum took place during the American Association for Cancer Research’s Annual Meeting 2021, which had more than 13,500 registrants.
April 15: A NIAID-sponsored clinical trial closes to enrollment. The study was examining the efficacy of two drug combinations—baricitinib plus remdesivir or dexamethasone plus remdesivir—to treat hospitalized patients with COVID-19 on supplementary oxygen. The DSMB, overseeing the trial, determined that neither treatment protocol studied is likely significantly better than the other.
April 15: NIH announces that it is awarding up to $33 million over two years to fund nationwide projects that build evidence on safely returning students, teachers, and support staff to in-person school in areas with vulnerable and underserved populations. The awards are part of the NIH Rapid Acceleration of Diagnostics Underserved Populations program, which aims to increase COVID-19 testing access and uptake for vulnerable and underserved populations.
April 16: [https://employees.nih.gov/pages/coronavirus/all-staff-email-20210416.aspx] Francis Collins continues to encourage staff to use the free SARS-CoV-2 testing available at NIH to mitigate workplace transmission.
April 16: NIH scientists find that the experimental antiviral drug MK-4482 significantly decreases viral particles and disease damage in the lungs of hamsters treated for SARS-CoV-2 infection. Unlike the antiviral remdesivir (which is administered intravenously), MK-4482 can be delivered orally and is now in phase 2 and 3 human clinical studies. (Emerg Microbes Infect 9:2673–2684, 2020; DOI:10.1080/22221751.2020.18581770)
April 19: NIH announces it will provide $155 million in funding for a clinical trial to test several existing prescription and over-the-counter medications that treat symptoms of COVID-19. The large phase 3 trial aims to provide evidence-based self-administered treatment options for adult patients with COVID-19 who have mild-to-moderate symptoms and are not sick enough to be hospitalized.
April 21: A NIAID-sponsored phase 2 and 3 clinical trial launches to evaluate a new antibody used to treat SARS-CoV-2. Called SAB-185, the therapeutic is made from polyclonal human immune cells and will be tested on nonhospitalized people with mild or moderate cases of COVID-19.
April 22: Other trials for therapeutics that treat COVID-19 are underway this week. A phase 3 clinical trial, supported by NIAID and NHLBI, has begun enrollment to test the safety and efficacy of two therapeutics for treating severe COVID-19 in hospitalized patients. The trial is part of the ACTIV public-private partnership.
April 23: Participants begin enrolling at the NIH Clinical Center for a new study assessing how people with immune system deficiencies or dysregulations respond to COVID-19 vaccination. The NIAID-led study aims to enroll 500 people, 400 with primary or secondary immune system disorders, and 100 without such conditions.
April 28: NIH Director Francis Collins testifies before the House Energy and Commerce Health Subcommittee on the impact of post-acute sequelae of COVID-19, generally known as long COVID. He highlights NIH’s new research initiative to better understand this condition and find ways to treat or prevent it.
April 29: NIH announces it will fund an additional $29 million in grants for the NIH Community Engagement Alliance Against COVID-19 Disparities (CEAL) with $15 million to strengthen research within existing 11 teams and $14 million to fund 10 new research teams.. The funding is supported by the American Rescue Plan and bolsters research to help communities disproportionately affected by COVID-19. “The goal of this effort is to foster community-engagement research in communities [that] have been hit hardest by the pandemic,” said NHLBI Director Gary Gibbons.
April 30: In his email message to staff, NIH Director Francis Collins reports that over 60% of NIH employees have been vaccinated against COVID-19. He calls attention to a new study of the Pfizer and Moderna mRNA vaccines that found fully vaccinated adults 65 years and older, who are among the most vulnerable groups, were 94% less likely to be hospitalized than their unvaccinated counterparts.
This page was last updated on Monday, February 14, 2022
