Return of a Century-old Therapy for Fighting Life-threatening Antibiotic-resistant Infections
Paul Turner’s and Matthew Laub’s WALS Lectures on Phage Therapy
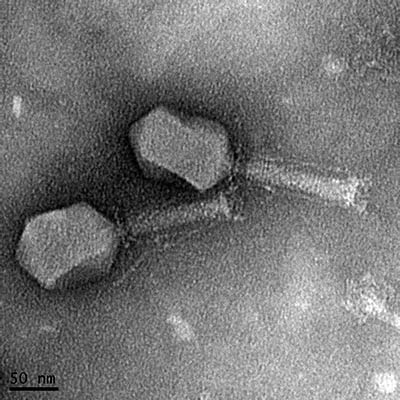
CREDIT: MANOJ RAJAURE, NCI
Transmission electron micrograph of a bacteriophage isolated from sewage. The phage kills a pathogenic strain of E. coli isolated from a patient with a urinary tract infection.
Antibiotic resistance (AR) looms as one of the biggest public health crises of our time. AR occurs when microbes such as bacteria develop the ability to evade the drugs designed to kill them. The Centers for Disease Control and Prevention estimate that over 2.8 million people get an AR infection in the United States each year, resulting in more than 35,000 deaths. AR is rising to dangerous levels worldwide while the pipeline of new antibiotics is running dry, threatening our ability to treat common infectious diseases. But the use of bacteriophages (phages) to treat AR infections caused by bacteria offers a glimmer of hope. Phages are naturally-occurring viruses that invade bacterial cells and replicate. The viral particles eventually burst out, killing the host cell in the process. Phages have coevolved with bacteria and function to keep microbe populations in check: Potency, self-amplification, and specificity make phages an attractive alternative to antibiotics. And phages are everywhere; one can assuredly find them in a lake, sewage water, or farm.
A promising treatment resurfaces
Phage treatment, known as phage therapy, has a colorful history going back a century. After the dawn of small-molecule antibiotics however, interest in the therapy quickly died down, at least in Western medicine. The exciting news is that phage therapy is making a comeback; recent case studies have been successful in using phages as an experimental therapy.
The therapy still has a long way to go as it is not yet generally approved by the FDA. And, as with antibiotics, bacteria also develop phage resistance. The evolutionary arms race between phages and bacteria has been going on for billions of years—which is critical to understand for phage therapy to become a success. Two experts, Paul Turner from Yale School of Medicine (New Haven, Connecticut) and Michael Laub from MIT (Cambridge, Massachusetts), shared their research on the evolutionary interaction between phages and bacteria and its significance to phage therapy at the NIH Director’s Wednesday Afternoon Lecture Series (WALS) on March 10 and March 17 respectively.
Evolutionary trade-offs

Paul Turner (Yale School of Medicine)
Turner is the Rachel Carson Professor of Ecology and Evolutionary Biology at Yale and a prominent expert on evolutionary trade-offs (he earned his Ph.D. with Richard Lenski, famous for his ongoing 33-year-old long-term evolution experiment on bacteria). A trade-off occurs when natural selection improves one trait at the expense of another. Turner shared his findings that demonstrated how evolutionary trade-offs between AR and phage resistance could be used in phage therapy.
His research team isolated phages that enter a bacterial host by selectively targeting the elements used by the bacteria to develop AR. The idea was simple: If the bacteria evolve phage resistance by modifying these elements, it will compromise their antibiotic resistance, making the bacteria susceptible to antibiotics once again. A synergistic treatment using phages together with antibiotics would leave little chance for the antibiotic-resistant bacteria to escape.
Bacteria often develop AR by expelling drugs through proteins known as efflux pumps. Turner showed how a phage that binds to an efflux pump of Escherichia coli (E. coli) results in the selection of phage-resistant bacteria that are now sensitive to tetracycline. A similar approach could target virulence factors. For example, his team found a phage that invades Pseudomonas aeruginosa by attaching to pili, hair-like appendages on the surface of bacteria to help in movement and surface adherence during infection. When the bacterium developed phage-resistance by losing the pili, it became avirulent (more vulnerable to the immune system) due to its inability to form biofilms and to move around.
“But there is a cautionary tale, and you should know what you are doing before using phages for therapy,” Turner said. Sometimes, instead of causing a trade-off, mutations cause a trade-up; they confer resistance to both antibiotics and phages.
The last part of Turner’s talk focused on emergency phage therapy. His team was granted FDA approval to treat a patient who developed a chronic AR infection after undergoing an aortic arch replacement. They chose a phage that used an efflux pump for entering the bacteria and predicted that the phage would kill the existing microbe population while exerting selection pressure on the bacteria to develop phage resistance, compromising their ability to maintain AR. Consistent with the evolutionary trade-off theory, a single application of the phage and a previously ineffective antibiotic (ceftazidime) resolved the infection with no sign of recurrence.
In another case, a 22 year-old patient with cystic fibrosis undergoing pulmonary failure had a high concentration of AR bacteria in her sputum. After a phage therapy treatment given via nebulizer, her lung function improved significantly. Interestingly, the treatment resulted in a population of bacteria that became sensitive to almost all antibiotics, opening the door to previously unavailable treatment options.
Turner is hopeful that phage therapy will be used in clinics soon. To date, Yale New Haven Hospital has successfully treated over 13 patients with AR infections using the emergency therapy. And things are moving in the right direction; The hospital was recently granted FDA approval to begin phage-therapy clinical trials.
From proteins to phages

Michael Laub (MIT)
Laub is a professor of biology at Massachusetts Institute of Technology and a Howard Hughes Medical Institute Investigator. He studies the coevolution of proteins and the selective pressures that drive the evolution of bacterial signaling pathways (mechanisms that allow bacteria to process information and respond to environmental changes). But “the coevolution of proteins is dear to my heart”, he said.
Laub’s ongoing work on the coevolution of toxin-antitoxin (TA) protein pairs (referred to as TA systems in bacteria) led to his foray into the world of phages. A TA system consists of a toxin gene and an associated antitoxin. The toxin is always a protein, and the antitoxin can be either a protein or a noncoding RNA. These systems are widespread in the bacterial kingdom, but why bacteria carry them remains a mystery. Laub's team discovered that cloning the TA systems found in several E. coli strains into E. coli K12 (a widely used laboratory strain of the bacterium) resulted in phage resistance.
Interestingly, the bacteria did not develop broad phage resistance, suggesting that TA systems seem to provide tailored protection only against specific phages. For example, one of the TA systems prevents the production of new viral particles by destroying the phage RNA. These findings establish TA systems in bacteria as another arm of immunity against phages in the ongoing evolutionary arms race.
“Phages fight back,” Laub said. His team identified a phage variant that evolved a counter-defense against one of the TA systems. This phage amplified a region of its DNA that encoded a protein capable of neutralizing the toxin, allowing the phage to propagate.
Both Laub and Turner are exploring concepts with far-reaching implications in phage therapy. Turner also believes that his work may help answer some bigger questions, such as how do we predict the emergence of viruses in new hosts? The investigation into evolutionary trade-offs between phages and bacteria might just reveal some clues.
To view a videocast of Turner’s WALS lecture “Phage Therapy to Combat Infections by Antibiotic-Resistant Bacteria,” delivered on March 10, 2021, go to: https://videocast.nih.gov/watch=41443.
To view a videocast of Laub’s WALS lecture “Innate Immunity for Bacteria Against Phage,” delivered on March 17, go to https://videocast.nih.gov/watch=41451 (HHS only).

Subhash Verma is research fellow in NCI’s Laboratory of Molecular Biology. In his spare time, he enjoys exploring nature, playing cricket and volleyball, and biking.
This page was last updated on Monday, February 14, 2022
