Research Briefs
Read about NIH scientific advances and discoveries by intramural scientists:
NCI: ENGINEERED IMMUNE CELLS MAY PREVENT CANCER SPREAD
NINDS, NCI, NIAMS, NICHD, NEI: DNA DAMAGE “HOT SPOTS” DISCOVERED WITHIN NEURONS
NIAID: NEW WAY TO TEST TREATMENTS FOR DEADLY BRAIN DISEASE
NICHD: CAFFEINE CONSUMPTION DURING PREGNANCY MAY LEAD TO SMALLER BIRTH SIZE
NIDA: ADOLESCENTS MAY DEVELOP ADDICTIONS FASTER THAN YOUNG ADULTS
NIAID, NCI: BACTERIOPHAGE TREATMENT RESCUES MICE FROM MULTI-DRUG RESISTANT KLEBSIELLA PNEUMONIAE
NHLBI,CC: CIRCULATING MITOCHONDRIAL DNA TRIGGERS INFLAMMATION IN SICKLE CELL DISEASE
NIEHS: MOSQUITO PROTEIN MAY INHIBIT SOME DANGEROUS VIRUSES
NIEHS: PROGESTERONE BALANCE IS KEY TO PRETERM BIRTH AND PROLONGED LABOR
NIEHS: POSSIBLE MECHANISM FOR DEVELOPMENT OF AUTOIMMUNITY
NCI: ENGINEERED IMMUNE CELLS MAY PREVENT CANCER SPREAD
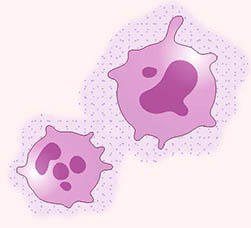
CREDIT: NCI
NCI scientists have genetically engineered myeloid cells (pink) to deliver an anticancer signal (purple dots) to sites where cancer may spread.
Scientists at NCI’s Center for Cancer Research have developed a form of immunotherapy that prevents and slows the spread of cancer, or metastasis, in mice. Researchers studied mice implanted with rhabdomyosarcoma, a type of cancer that is known to develop in the muscles and spontaneously metastasize to the lungs. The NCI team first examined immune cell changes in the lungs during the period before metastasis but after muscle-tumor development. They found that several immune cell types—in particular myeloid cells—were attracted to the premetastatic site. However, instead of sending signals to recruit cancer-fighting immune cells (such as T-cells), the myeloid cells were actively suppressing the immune response.
The researchers genetically engineered myeloid cells (GEMys) to produce interleukin 12 (IL-12), a protein known to signal and activate a cancer-fighting immune response. “We chose myeloid cells to deliver IL-12 based on their unique ability to home [in on] tumors and metastatic sites,” said the study’s leader, Rosandra Kaplan. “With IL-12, we’re turning the volume up on a message that’s been quieted.”
The results proved remarkable: Mice with rhabdomyosarcoma that were treated with GEMys had decreased metastatic cancer and muscle-tumor size, and they lived longer than control mice. Similar results were observed when the immunotherapy was applied to an aggressive pancreatic cancer that metastasized to the liver. Additionally, GEMys in combination with other treatments, such as chemotherapy, improved treatment outcomes and even prevented cancer recurrence.
As a final step, Kaplan’s team created GEMys from human cells grown in a lab and plans to test the safety of human GEMys in a clinical trial of adults with cancer. (NIH authors: S. Kaczanowska, D.W. Beury, V. Gopalan, A.K. Tycko, H. Qin, M.E. Clements, J. Drake, C. Nwanze, M. Murgai, Z. Rae, W. Ju, K.A. Alexander, J. Kline, C.F. Contreras, K.M. Wessel, S. Patel, S. Hannenhalli, M.C. Kelly, and R.N. Kaplan, Cell 184:2033–2052.e21, 2021; DOI:10.1016/j.cell.2021.02.048)
NINDS, NCI, NIAMS, NICHD, NEI: DNA DAMAGE “HOT SPOTS” DISCOVERED WITHIN NEURONS
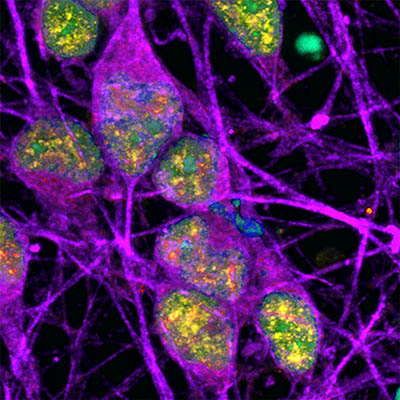
CREDIT: NINDS
Neurons (labeled in purple) show signs of an active DNA repair process (labeled in yellow). The cells’ DNA itself is labeled in cyan (in this image, overlap between cyan and yellow appears green).
Researchers at NIH and the University of Sussex (Falmer, England) have identified specific regions, or “hotspots,” in neuron genomes that appear to accumulate DNA damage known as single-strand breaks (SSBs)—a finding that has the potential to reshape the way we think about DNA damage and its role in neurobiology.
The scientists found that the most prominent concentrations of SSBs localized to distinct regions of DNA called enhancers, which control nearby gene activity. One way a cell can influence gene expression (turning a gene on or off) is by applying a chemical tag known as a methyl group to specific sites on its DNA; removal of this tag typically allows the gene to be activated. Further investigation of these enhancers revealed that SSBs accumulated when the methyl group had been removed.
The researchers proposed that the absent methyl group resulted in the formation of SSBs. And, at least in neurons, the failure to properly repair DNA damage, not the damage itself, could dysregulate gene expression, thereby contributing to the development of neurodegenerative diseases. (NIH authors: W. Wu, S.E. Hill, W.J. Nathan, J. Paiano, E. Callen, D. Wang, K. Shinoda, N. van Wietmarschen, J.M. Colón-Mercado, D. Zong, R. De Pace, H. Shih, S. Coon, M. Parsadanian, R. Pavani, S. Park, S.K. Jung, C. Chen, R. Casellas, M.E. Ward, and A. Nussenzweig, Nature 2021; https://DOI.org/10.1038/s41586-021-03468-5)
NIAID: NEW WAY TO TEST TREATMENTS FOR DEADLY BRAIN DISEASE
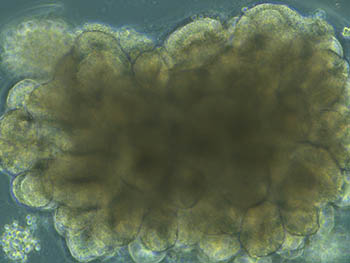
CREDIT: NIAID
A cerebral organoid shown overlaid with test results from prion infected organoids that were left untreated or treated with PPS. The results show that treatment reduces disease-associated protein. Shown: Brightfield microscope image of an organoid during development, showing highly structured regions forming.
NIAID scientists have recently developed a new way to test treatments for Creutzfeldt-Jakob disease (CJD). CJD is a fatal, incurable, neurodegenerative brain disease caused by infectious prion proteins. Prion diseases occur when a prion misfolds into a pathogenic form and spreads to neighboring neural tissue. CJD may arise spontaneously, result from a hereditary mutation within the prion gene, or occur due to infection, such as from eating contaminated meat products. The lack of a human model to test drugs for CJD has been a barrier to developing treatments; studies in mice have failed to identify antiprion compounds that were ultimately effective in humans.
The NIAD team had previously established a human cerebral organoid system—a small cluster of human brain cells grown in a lab from skin cells. The cerebral organoids have similar organization, structure, and electrical signaling to human brain tissue; they have been shown to accurately predict neurotoxicity—and therapeutic benefits—of drug treatments in humans.
The researchers infected the cerebral organoids with pathogenic prions and were able to observe the spread of the misfolded proteins. Scientists then tested pentosan polysulfate (an established antiprion compound), which successfully delayed prion propagation when applied both prophylactically and after an established infection. The findings demonstrate the utility of cerebral organoids as a model for screening therapeutic drug candidates to treat human prion diseases.(NIH authors: B.R. Groveman, N.C. Ferreira, S.T. Foliaki, R.O. Walters, C.W. Winkler, B. Race, A.G. Hughson, and C.L. Haigh, Sci Rep 11:Article number 5165, 2021; DOI:10.1038/s41598-021-84689-6)
NICHD: CAFFEINE CONSUMPTION DURING PREGNANCY MAY LEAD TO SMALLER BIRTH SIZE
Should pregnant women be consuming caffeine at all? Recent findings published by NICHD researchers suggest they should not. The study found that women who consumed as little as the equivalent of a half cup of coffee a day (50 milligrams) had slightly smaller babies than women who did not drink caffeinated beverages.
The study enrolled more than 2,100 nonsmoking women from racially and ethnically diverse backgrounds with no history of health problems before pregnancy. The women reported their daily consumption of caffeinated beverages and provided a blood sample between 10 and 13 weeks of pregnancy. Researchers then analyzed the plasma samples for evidence of caffeine metabolism and caffeine concentration.
Compared with those consuming minimal or no caffeine, women with the highest plasma caffeine concentrations gave birth to babies that were 84 grams lighter and 0.44 centimeters shorter, and that had head circumferences that were 0.28 centimeters smaller. Likewise, babies born to women who consumed about 50 milligrams of caffeine a day were 66 grams lighter than babies born to non-caffeine consumers.
The long-term implications of the findings are unclear. But researchers note that caffeine has been hypothesized to restrict blood supply to the fetus, inhibiting growth and potentially disrupting fetal stress hormones, putting infants at risk for childhood obesity and chronic diseases later in life. (NIH authors: J.L. Gleason, F. Tekola-Ayele, R. Sundaram, S.N. Hinkle, Y. Vafai, G.M. Buck Louis, M. Amyx, A.M. Bever, M.M. Smarr, and K.L. Grantz, JAMA Netw Open 4(3):e2132382021;.DOI:10.1001/jamanetworkopen.2021.3238)
NIDA: ADOLESCENTS MAY DEVELOP ADDICTIONS FASTER THAN YOUNG ADULTS
In a recent study published in JAMA Pediatrics, researchers at NIDA reported that the onset of substance-use disorders (SUDs) were associated with the age an individual tried a drug for the first time.
The scientists analyzed data from participants (ages 12–25 years) of the National Surveys on Drug Use and Health, from 2015 to 2018. They compared the prevalence of substance use across two age groups: adolescents (ages 12–17) and young adults (ages 18–25). Each group was examined at four time intervals after their first use of a drug: less than 12 months, 12–24 months, 24–36 months, and more than 36 months. The study found that adolescents have a 10.7% chance of developing a SUD to cannabis within 12 months of their first experience, compared with 6.4% in young adults. This difference grew to 20.1% in adolescents and 10.9% in young adults in the over-36-month interval. Similarly, an overall higher rate of SUDs were found in adolescents across all four timepoints with the misuse of prescription drugs such as opioids, stimulants, and tranquilizers.
In contrast, the dependency on nicotine and alcohol after 12 months was significantly higher in young adults compared with adolescents. The young adult group also showed a high prevalence of heroin SUD (30.9%) and methamphetamine SUD (24.8%) after one year.
“Adolescents may develop addiction to substances faster than young adults. This study provides further evidence that delaying substance exposure until the brain is more fully developed may lower the risk for developing a substance use disorder,” said Nora Volkow, lead author of the study. (NIH authors: N.D. Volkow, B. Han, E.B. Einstein, and W.M. Compton, JAMA Pediatr e206981, 2021; DOI:10.1001/jamapediatrics.2020.6981)
NIAID, NCI: BACTERIOPHAGE TREATMENT RESCUES MICE FROM MULTI-DRUG RESISTANT KLEBSIELLA PNEUMONIAE
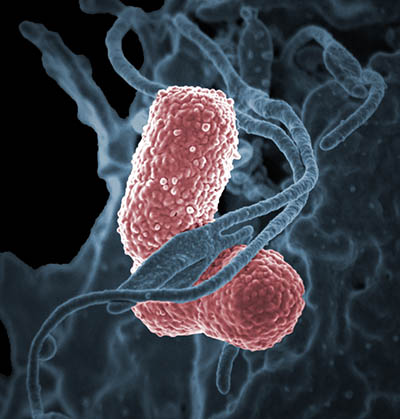
CREDIT: NIAID
Colorized scanning electron micrograph showing carbapenem-resistant Klebsiella pneumoniae (pink) interacting with a human neutrophil.
Scientists from NIAID’s Rocky Mountain Laboratories (Hamilton, Montana) and NCI have developed an effective therapy using bacteriophages (phages, viruses that infect and kill bacteria) against multidrug-resistant Klebsiella pneumoniae sequence type 258 (ST258) in mice. The bacterium is a threat to hospitalized patients and causes high rates of mortality when left untreated. Although newer antibiotics have shown efficacy against ST258, resistance against these drugs is expected to increase and the development of novel treatments is essential.
Interest in using phages as a promising therapeutic for antibiotic resistance has increased in recent years. The authors found that treating acute sepsis with two phages (phage P1 and phage P2) that target ST258 improved survival: Mice were first injected with ST258 to induce infection. They were then treated at several different times after infection with phage P1, phage P2, or both. Survival outcomes were dramatically improved from 0% in saline-treated control mice to 100% in mice treated with both phages. Early treatment combined with using both phages resulted in the strongest recovery.
The authors noted that although lower bacterial concentrations were seen in the blood and tissues of the phage-treated mice, the recovered ST258 bacterium began to demonstrate phage resistance. Further work on the strategic selection of phages, phage dosing, and engineering may improve the ability to predict therapeutic efficacy of phage treatment in the future. (NIH authors: S. Hesse, N. Malachowa, A.R. Porter, B. Freedman., S.D. Kobayashi, D.J. Gardner., D.P. Scott, S. Adhya, and F.R. DeLeo, mBio 12:e00034-21, 2021; DOI:10.1128/mBio.00034-21)
NHLBI,CC: CIRCULATING MITOCHONDRIAL DNA TRIGGERS INFLAMMATION IN SICKLE CELL DISEASE
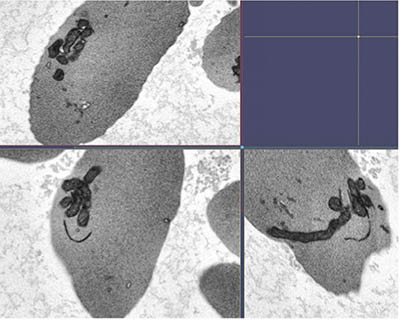
CREDIT: SWEE LAY THEIN, NHLBI
Scanning electron microscopy image of mitochondrial bundles in several sickle cell red blood cells, showing evidence that circulating red blood cells from people with sickle cell disease abnormally retain mitochondria.
Sickle cell disease (SCD) is an inherited disorder caused by a mutation in hemoglobin, the oxygen-carrying protein found in red blood cells. The abnormal hemoglobin causes red blood cells to distort into a sicklelike shape. This can block blood vessels, resulting in pain, inflammation, and organ damage.
In a recent study published in Blood, a team from NHLBI’s Sickle Cell Branch analyzed plasma concentrations of free-floating DNA from mitochondria, an organelle that generates chemical energy within a cell. The researchers identified elevated mitochondrial cell-free DNA (cf-mtDNA) in the plasma of SCD patients compared with healthy control subjects. Normally, mature red blood cells do not contain mitochondria, but those of SCD patients appear to retain the organelle.
The authors discovered that the resulting increase in cf-mtDNA triggered an immune response, marked by the formation of neutrophil extracellular traps (NETs). NETs are a network of fibers critical in combatting infection, but their formation in response to cf-mtDNA in SCD can contribute to the chronic inflammation experienced by patients.
These findings indicate that cf-mtDNA is not only a prognostic biomarker but may also contribute to the pathology of SCD, opening new avenues for investigation and therapeutic intervention. (NIH authors: L. Tumburu, S. Ghosh-Choudhary, F.T. Seifuddin, E.A. Barbu, S. Yang, M.M. Ahmad, L.H.W. Wilkins, I. Tunc, I. Sivakumar, J.S. Nichols, P.K. Dagur, S. Yang, L.E.F. Almeida, Z.M. Quezado1, C. Combs, E.J. Lindberg, C.K.E. Bleck, J. Zhu, A.S. Shet, J.H. Chung, M. Pirooznia, and S.L. Thein, Blood 2020009063, 2021; DOI:10.1182/blood.2020009063)
NIEHS: MOSQUITO PROTEIN MAY INHIBIT SOME DANGEROUS VIRUSES
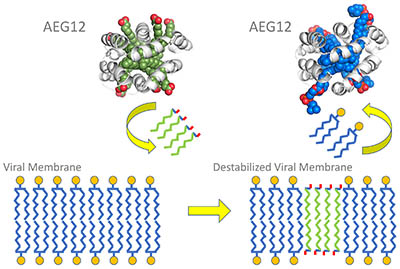
CREDIT: GEOFFREY MUELLER, NIEHS
The viral membrane lipid bi-layer has water-loving heads (represented by the small circles) and water-avoiding tails (blue squiggles). AEG12 (in green and gray) inserts some of its lipids (green squiggles) into the viral membrane, destabilizing it. During this exchange, AEG12 incorporates viral lipids into its interior (blue and gray).
The mosquito protein AEG12 strongly inhibits flaviviruses and weakly inhibits coronaviruses, according to NIEHS scientists and their collaborators. Flaviviruses cause yellow fever, dengue, West Nile, and Zika, among other illnesses. Mosquitoes produce AEG12 when they take a blood meal or become infected with flaviviruses. The researchers found that AEG12 destabilizes the viral envelope, which could lead to treatments for diseases that affect millions of people around the world. However, the protein does not affect viruses without an envelope, such as those that cause pink eye and bladder infections. AEG12 might also be effective against SARS-CoV-2, the coronavirus that causes COVID-19, although it will take years of bioengineering to make AEG12 a viable therapy, because it also breaks opens red blood cells. Researchers will have to find ways to limit the protein so it only targets viruses.
At the molecular level, AEG12 has high affinity for viral lipids and rips them from the viral envelope, giving it great killing power. NIEHS scientists used X-ray crystallography to discover the molecular structure of AEG12. Another approach used to define AEG12’s lipid preferences was nuclear magnetic resonance. The researchers subjected AEG12 molecules to a magnetic field and measured how the nuclei of the molecules spin or change frequency. (NIH authors: A.C.Y. Foo, P.M. Thompson, S.-H. Chen, B. Lupo, E.F. DeRose, S. Arora, V.D. Placentra, L. Perera, L.C. Pedersen, N. Martin, and G.A. Mueller, Proc Natl Acad Sci USA 118:e2019251118, 2021; DOI:10.1073/pnas.2019251118)
NIEHS: PROGESTERONE BALANCE IS KEY TO PRETERM BIRTH AND PROLONGED LABOR

CREDIT: PROSTOCK-STUDIO/SHUTTERSTOCK.COM
Preterm birth and prolonged labor can affect the health of the mother, the infant at birth, and the infant after birth, according to NIEHS researchers.
Unbalanced progesterone signals influence preterm and prolonged labor, according to new research led by NIEHS scientists. During pregnancy, progesterone receptor types A and B (PGR-A and PGR-B) signaling helps to prevent the uterus from contracting and going into labor prematurely. In this first-of-its-kind study, the scientists showed how unbalanced PGR-A and PGR-B signaling can affect pregnancy duration. The team found that PGR-A promotes muscle contraction and PGR-B prevents such contraction and identified biological pathways influenced by both molecules. This study revealed that the relative abundance of PGR-A and PGR-B may be critical in promoting healthy pregnancy. The public-health implications are significant.
Progesterone treatment aimed at preventing premature labor can help a subset of patients, but for other individuals, confounding factors may reduce effectiveness. The research team found novel molecules that control uterine muscle contraction and could serve as future therapeutic targets. The findings may also help to advance treatment for prolonged labor. Novel proteins identified as being part of progesterone signaling could serve as a key molecular switch of uterine contraction through drug-dependent regulation of their activities. (NIH authors: S.-P. Wu, R. Li, J. Liu, O.M. Emery, T. Wang, L. Zhou, and F.J. DeMayo, Proc Natl Acad Sci USA 118(11):e2011643118, 2021; DOI:10.1073/pnas.2011643118)
[BY JESSE SAFFRON, NIEHS]
NIEHS: POSSIBLE MECHANISM FOR DEVELOPMENT OF AUTOIMMUNITY
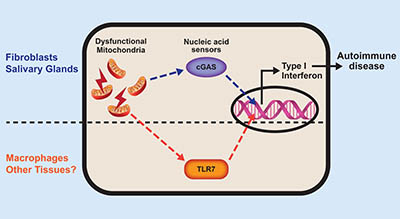
CREDIT: PRASHANT RAI, NIEHS
The nucleic acid that spills out of broken mitochondria activates tissue-specific sensors. These processes lead to type 1 interferon production and eventually autoimmune disease.
NIEHS researchers found that an IRGM1 knockout mouse strain developed an autoimmune disease that looks like Sjögren syndrome in humans. These mice also produced lots of the inflammatory protein, type 1 interferon. The scientists determined the mice suffered from a condition that appeared to be caused by the accumulation of defective mitochondria, which activated the immune system. Specifically, when damaged mitochondria release their DNA and RNA into the interior of the cell, immune sensors detect these nucleic acids as foreign, triggering type 1 interferon production. The article was published in Nature Immunology and suggests that the inability to remove damaged mitochondria may cause Sjogren’s and other autoimmune diseases, like lupus, through production of interferon.
The scientists also determined that while various tissues in the knockout mouse strain made type 1 interferon as an immune reaction to damaged mitochondria, the mechanism of activation differed. In fibroblasts, leaking mitochondrial DNA activated the cGAS immune receptor, but in macrophages, mitochondrial RNA activated the TLR7 receptor. In short, cGAS caused autoimmune damage in some organs of the IRGM1-deleted mouse, but not others. (NIH authors: P. Rai, K. Janardhan, J. Meacham, J. Madenspacher, W-C Lin, P.W.F. Karmaus, J. Martinez, and M.B. Fessler, Nat Immunol 22:312-321, 2021; DOI:10.1038/s41590-020-00859-0)
This page was last updated on Monday, February 14, 2022
