2017 Research Festival: Plenary Sessions
BRAIN Initiative: Bridging Gaps in Neuro Knowledge
Plenary Session I
PHOTO CREDIT: PHONLAMAI PHOTO/THINKSTOCKPHOTOS.COM
The BRAIN Initiative funds intramural and extramural scientists who create new technologies to change the way neuroscience studies the brain.
The Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative was launched in 2013 to develop new methods and to bridge the knowledge gaps for diagnosing and treating mental-health diseases and neurological disorders. It funds scientists who create new technologies to change the way neuroscience studies the brain, explained Gregory Farber (NIMH), co-leader of the BRAIN initiative coordination team and one of the presenters at the first plenary session held on September 13 at the 2017 NIH Research Festival.
Through the BRAIN Initiative, NIH is providing grants to both extramural and intramural researchers and widening its net to draw in mathematicians, physicists, and computer scientists. These investigators are first-time NIH applicants from outside of the traditional neuroscience field, and they will change the way biomedical research is conducted. The long-term goal is to “make circuit abnormalities the basis of diagnostics, and normalization of circuit function the target of intervention,” according to the BRAIN Initiative website. Three grant awardees presented their work at the plenary session.
BRAIN-grantee Dietmar Plenz (NIMH) described his studies of how neurons fire together in specific patterns, called “neuronal avalanches,” which can be described with computer modeling and mathematical algorithms. The activity of neurons and the earth’s seismic eruptions follow similar mathematical equations; Plenz’s group first described how these neuronal avalanches can be detected in a manner similar to how we use the Richter scale to measure earthquakes. His group hypothesizes that neuronal avalanches are a new type of brain activity that can be distinguished from network oscillations and may provide a means for the brain’s ability to optimize information processing while maintaining network stability.
Another BRAIN-funded investigator, Patrick Kanold (University of Maryland, College Park), and Plenz are collaborating to describe how groups of neurons fire together. They have discovered that small-sound stimuli evoke neuronal avalanches in the auditory part of the brain, complete with “aftershocks” of activity that carry stimulus information for many seconds. Kanold’s group focuses on the circuit interactions between cells in the rodent auditory system. His studies show that the auditory cortex organizes as a tonotopic map (arranged by pitch or sound frequencies) at large scale. However, this map becomes much more intermingled at the single-neuron level, indicating that our understanding of the brain’s organization may require precise measures at single-cell resolution, a major aim of the BRAIN Initiative.
Neuroscience “needs a collaborative effort of many kinds of people,” said Kanold. “We need some traditional neuroscientists. We need tool builders, people who build microscopes and genetic tools, computational neuroscientists and theorists,” he continued. “What it means is there’s a change in the way we do neuroscience: You have team science.”
Peter Basser (NICHD) described advances his group has made, using axon-diameter measurements, in detecting connectivity in the brain. Basser is best known for the development of diffusion tensor imaging, which allows for noninvasive imaging of brain connectivity by measuring white-matter tracts in both the clinic and the lab. White matter (myelin) wraps neurons and forms a type of insulation to make sure signals get where they’re going quickly, and it also reduces alterations in the signals’ strength and timing. Using his BRAIN grant, Basser’s group is developing a latency map to measure how long it takes to send “packets of information” from one place to another. Disruptions in white matter can cause problems in how the brain talks within itself and to the rest of the body. These problems can result in paralysis, cognitive and motor dysfunction, and sometimes death. Scanning for myelin health is a new tool that clinicians can use to diagnose demyelinating diseases such as multiple sclerosis.
“There is this enormous mystery waiting to be unlocked,” said President Barak Obama when announcing the initiative in 2013. “The BRAIN Initiative will change that by giving scientists the tools they need to get a dynamic picture of the brain in action and better understand how we think and how we learn and how we remember. And that knowledge could be, will be, transformative.”
The Plenary Session “The BRAIN Initiative,” held on September 13, 2017, was moderated by Dietmar Plentz (NIMH). To watch a videocast, go to https://videocast.nih.gov/launch.asp?23456. Intramural researchers wishing to find out more about how to apply for BRAIN grants can visit https://www.braininitiative.nih.gov/funding/index.htm.
Inflammatory Diseases: Fixing Things When the Immune System Goes Awry
Research Festival Plenary Session II

CREDIT: ZERBOR/THINKSTOCKPHOTOS.COM
At the turn of the 21st century, the immune system, while complicated, seemed fairly straightforward: Immune cells, both innate and adaptive, fought off infections, and non-immune cells had non-immune functions. But in the past 17 years, our understanding of the immune system has radically changed. For inflammatory diseases, diseases in which the regulation of the immune system is fundamentally altered, we have come to see that non-immune cells—and the microbiota as well—have critical influence on the course of disease.
“We see now that on many levels, all cells are immune cells,” said Susan Amara (NIMH), co-chair of the 2017 NIH Research Festival. In the festival’s second plenary session, the speakers highlighted how unexpected regulation and functions of the immune system have led to a new understanding of immune deficiencies, autoimmune diseases, and cancer therapeutics.
In the first presentation, given by William Comrie (NIAID), a postdoctoral fellow in Michael Lenardo’s lab, the lab’s research into rare, congenital disorders of the immune system was discussed. Using next-generation sequencing, “you can learn the genetic cause of an unknown disease and potentially target that biological pathway,” said Comrie. “Along the way, we sometimes gain insight into key human biology.”
In one example, the researchers found that a congenital autoimmune disorder was caused by mutations in CTLA4, which codes for cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), a receptor critical for controlling T-cell activation; the mutations triggered runaway activation of T cells. A clinical trial that uses a CTLA-4 mimic to replace the missing signal is currently underway at the NIH Clinical Center. In a second example, Comrie described a new primary immune deficiency characterized by early-onset protein-losing enteropathy, gastrointestinal inflammation, and deadly vascular thrombosis. Screening of 11 patients in eight families revealed that the disorder was caused by mutations in both copies of the gene encoding cluster of differentiation 55 (CD55), which prevents complement-mediated attack of human cells. The elevated complement activation and elevated terminal membrane-attack-complex formation in patients appear to be the root cause of much of the intestinal and vascular damage, because treatment with complement inhibitors in CD55-deficient patients greatly reduces the symptoms of disease.
In the second presentation, Romina Goldszmid (NCI) explored the role of inflammation and the tumor microenvironment in cancer–and not only on the development of cancer, but also on a patient’s response to standard chemotherapies. Tumors, Goldszmid said, can be classified immunologically as “hot” (inflamed, with high numbers of infiltrating effector immune cells), “cold” (not inflamed, with few infiltrating effector immune cells and more cells with immunosuppressive activity), or somewhere in-between. Hot tumors with a high concentration of CD8+ T cells are more likely to respond to immunotherapies (therapies based not on small-molecule drugs, but on antibodies or specially adjusted immune cells).
Surprisingly, hot tumors are also more likely to respond better to standard chemotherapy treatments. But the mechanism of the chemotherapy response is different: It requires the action of innate myeloid cells. Goldszmid found that, if primed by the proper microbiota, neutrophils can release reactive oxygen species after encountering preliminary damage from certain chemotherapeutic agents, thus intensifying the anticancer effect of the therapy. The key, said Goldszmid, is “thinking about how the therapies work” and understanding whether they need help from cells like neutrophils and the microbiota to be effective. Getting the right balance of signals to harness the power of the immune system may be the key to cancer treatment, whether the treatment is chemotherapy or immunotherapy.
Mariana Kaplan (NIAMS), the third speaker in the plenary session, also found neutrophils to be unexpectedly critical in her study of autoimmune disorders such as systemic lupus erythematosus (SLE, or lupus). While SLE is generally thought to be mediated by antibodies reactive to autoantigens (abnormal self-antigens) such as DNA, Kaplan sought to answer questions such as how the autoantigens were being released and what was causing complications such as atherosclerosis, which is also common in people with SLE. She found a key role for neutrophils in this process: When activated by certain danger signals, neutrophils undergo a process known as NETosis (NET stands for “nuclear extracellular traps”) in which the neutrophils extrude their nuclear material along with molecules present in their granules, making them available to antigen-presenting cells and activating a variety of inflammatory pathways and vascular damage. SLE patients, she found, have a high concentration of neutrophils predisposed to undergoing NETosis. “This gives us some hope that if we target some of these deleterious pathways mediated by these neutrophils, we may be able to prevent or mitigate some of the organ damage” in SLE patients, said Kaplan.
“No matter what disease you are working on,” said session moderator John J. O’Shea (NIAMS), “you may not think of yourself as an immunologist or an inflammatologist, but in fact you are.” As shown by the speakers in the plenary session, the interplay between immune cells, non-immune cells, and the microbiota affects the body in unexpected ways. “Every cell,” O’Shea emphasized, “is an immune cell.”
To watch a videocast of the 2017 Research Festival’s “Inflammatory Diseases” plenary session, held on September 14, 2017, go to https://videocast.nih.gov/launch.asp?23459.
The Cancer Moonshot
2017 Research Festival Plenary Session III
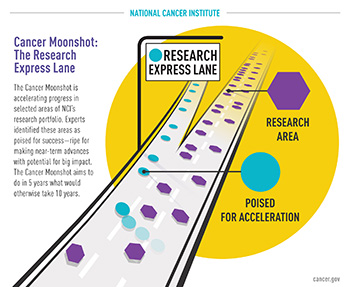
CREDIT: NCI
Illustration showing that the Cancer Moonshot is similar to an express lane on a highway when it comes to accelerating cancer research.
The Cancer Moonshot, announced by former President Barack Obama in his last State of the Union Address in January 2016, aims to double the rate of progress in our understanding of cancer and achieve in five years what would otherwise take a decade. Plenary session moderator Dinah Singer (NCI-CCR) outlined the main goals of the initiative:
- Accelerate our understanding of cancer across the cancer continuum from basic to translational and clinical, including prevention and screening.
- Encourage more collaboration and cooperation across the entire cancer-research community, not just NIH-funded research.
- Improve and enhance data sharing.
What does the initiative mean for intramural researchers? Plenty. Singer explained how a blue-ribbon panel of 28 scientific experts (with the help of 150 working-group members) developed 10 scientific recommendations, which are already being implemented. Several new extramural and intramural initiatives will be funded, including three intramural projects being launched as part of the Cancer Moonshot and described at this plenary session: a formal evaluation of single-dose regimens of the human papillomavirus (HPV) vaccines; population screening for cancer-predisposition genes; and a rare-tumor initiative.
HPV vaccines. Some types of the sexually transmitted HPV cause about 300,000 deaths from cervical and other cancers per year worldwide. The HPV vaccine—usually administered in two to three doses to adolescents and three doses to young adults—can provide protection, yet many people go unvaccinated, especially in low-income countries. Aimée Kreimer (NCI-DCEG) is testing one-dose vaccines versus two-dose vaccines to prevent HPV. A one-dose vaccine could dramatically lower the barriers to vaccination.
Kreimer described a new NCI HPV-vaccine trial that will be conducted in Costa Rica. The evidence to date suggests that although this subunit vaccine typically requires a multidose regimen, a single dose may produce durable protection against HPV infections. Subunit vaccines only contain antigens to and not live particles of the pathogen. The new trial will definitively answer the question whether one dose of the HPV vaccine is sufficient, and if so, it raises the potential for other subunit vaccines to be administered in single-dose regimens.
“For those of you who have small children, we know how many vaccine doses our children get in the first five years,” Kreimer said. “This is not even possible in developing countries, so think how we can revolutionize vaccinology if we demonstrate that a single-dose platform can work.”
Population screening for cancer-predisposition genes. There are more than 120 cancer-predisposition genes (CPG). Carriers of germ-line pathogenic variants in CPG are at a high risk for cancer, and preventive strategies may be implemented. Maria Isabel Achatz (NCI-DCEG) discussed her group’s previous experience in discovering the high occurrence of a founder mutation in the TP53 gene in Brazil involved in Li-Fraumeni syndrome, a rare inherited disorder that leads to a higher than normal risk of developing certain cancers. Defining the prevalence of the founder mutation in 0.3 percent of the population of southern Brazil’s population has public-health implications. Achatz’s group will be also measuring the population prevalence and cancer penetrance of germ-line pathogenic mutations in CPG in an unbiased cohort of newborn screening samples from Michigan. She hopes to identify the contribution of germ-line variants in CPG to childhood and young-adult cancer etiology and to understand how germ-line variants affect underrepresented minorities. All information they collect will be available in public databases for data queries, hypothesis generation, and future testing.
The Rare Tumors Initiative. Rare tumors lead to a quarter of cancer deaths and contribute greatly to our understanding of cancer mechanisms, explained Brigitte Widemann (NCI-CCR), who presented a proposal for the Rare Tumors Initiative and Rare Tumor Patient Engagement Network. She provided an overview of examples of NCI’s Center for Cancer Research’s intramural research in rare tumors. She highlighted research on chimeric antigen-receptor therapies for acute myeloid leukemia (a type of blood cancer), the development of the first therapy for plexiform neurofibroma (a benign tumor of the sheaths of peripheral nerves), clinical trials for targeted therapies for gastrointestinal stromal tumors (uncommon tumors of connective tissue in the gastrointestinal tract), and clinical data and tissue analysis for ependymoma (a rare tumor of epithelial lining of ventricles in the brain or spinal cord or, rarely, the pelvis).
The Rare Tumors Initiative aims to develop a shared infrastructure to study rare tumors; connect patients and investigators through advocacy groups; and develop a biorepository that will be available to researchers worldwide.
To view a videocast of the 2017 Research Festival Plenary Session III, “The Cancer Moonshot,” go to https://videocast.nih.gov/launch.asp?23462.
This page was last updated on Friday, April 8, 2022
