The Other Brain
Neuron-Glia Interactions
President Barack Obama’s Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative, launched April 2, 2013, promised to “accelerate the development and application of new technologies that will enable researchers to produce dynamic pictures of the brain that show how individual brain cells and complex neural circuits interact at the speed of thought.” At first, some neuroscientists were dismayed because the plan seemed focused on mapping neuronal connections. What about glia, the non-neuronal cells that make up most of the brain? There are about 100 billion neurons in the human brain and many more glia.
Fortunately, the NIH BRAIN working group that is creating a plan to accomplish the BRAIN Initiative’s goals recognized the importance of glial cells. In its interim report, issued in September 2013, the group identified several high-priority research areas including generating “an integrated, systematic census of neuronal and glial cell types.” The mention of glia was a huge step for the once neuron-centric field.
Although glial cells have traditionally been thought of as passive support cells in the nervous system, they are getting a starring role in the new Neuron-Glia Interactions (NGI) Scientific Interest Group (SIG). Glia, once considered to be simply the “glue” holding neurons in place, are proving to be diverse in origin, shape, and function. They are even being noted for their effect on functional activity and neural plasticity and their role in pathogenesis.
In fact, there are four major types of glia, all of which communicate with one another and with neurons via electrical and chemical signals: oligodendrocytes, astrocytes (or astroglia), microglia in the brain; and Schwann cells in the peripheral nervous system. Oligodendrocytes form the myelin sheath that insulates and provides metabolic support to axons in the brain. Schwann cells perform similar functions in nerves outside the brain. Astrocytes provide nutrients to neurons and regulate electrical signals. Microglia remove debris and provide a defense against infection. There are many other types, too, including satellite glial cells (similar to astrocytes); glia in the enteric nervous system (intrinsic nervous system of the gastrointestinal tract); and glia-like cells found in the peripheral nervous system such as in the inner ear.
JONATHAN COHEN, FDA
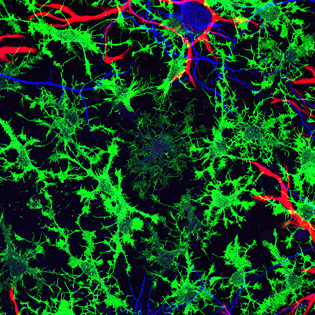
Glia, non-neuronal cells once considered to be simply the “glue” holding neurons in place, are now being noted for their effect on functional activity and neural plasticity and their role in pathogenesis. Shown here: The rat hippocampus in a dish; confocal image of immature oligodendrocytes (green), astrocytes (red), and neurons (blue), in cell culture at 40x magnification.
“Glia literally have their feet in everything,” said Amy Shafqat, a graduate student in the National Institute of Neurological Disorders and Stroke (NINDS), who was a driving force behind the creation of the NGI SIG. The SIG is co-moderated by senior investigators Michael O’Donovan (NINDS) and R. Douglas Fields (Eunice Kennedy Shriver National Institute of Child Health and Human Development, NICHD). O’Donovan hopes that the SIG will bring people together who are studying diverse functions of glia in different areas of the nervous system.
Fields, a longtime supporter of glial research, thinks neuron-glia interactions have been neglected. “Glia were overlooked for a century, but now people realize the brain is an organ, not [made up of a] single [type of] cell,” he said. “You need to understand all the cells.” He described the importance of including glia in the BRAIN Initiative in an editorial entitled “Map the Other Brain” (Nature 501:25–27, 2013).
Interest in the NGI SIG has been overwhelming, with more than 120 scientists participating. In fact, intramural research into neuron-glia interactions is widespread, spanning institutes. Although many NIH researchers are conducting glial research, they don’t all consider themselves glial biologists. The NIH Catalyst recently interviewed several of them. Excerpts of those interviews appear below.
Herbert Geller, a senior investigator in the National Heart, Lung, and Blood Institute (NHLBI), has been studying glia since 1984. “Glial cells are key to sustenance,” said Geller. Through glia “neurons grow and survive … behave and misbehave.” After spinal cord injury, astrocytes can form a glial scar and prevent regeneration of neurons. Geller’s laboratory studies the role of chondroitin sulfate proteoglycans, a family of molecules produced by astrocytes contributing to the glial scar.
R. Douglas Fields, NICHD’s chief of the Nervous System Development and Plasticity Section and editor-in-chief of the journal Neuron Glia Biology, also got excited about glia in the 1980s after a calcium-imaging experiment to look at neurons lit up non-neuronal cells as well. “It was a bewildering and exciting finding,” recalled Fields. Eager to see whether Schwann cells (glial cells in the peripheral nervous system) also responded, he tested his hypothesis at NIH in building 49. “We were seeing neurons fire with our own eyes,” he said. “The glial response was slow, but [glia] did respond.” His research has focused on myelinating glia that change neuronal insulation with damage or pathology and may regulate functional activity like learning.
Senior investigator Jau-Shyong Hong, at the National Institute of Environmental Health Sciences (Research Triangle Park, N.C.), heads the Neuropharmacology Group and is interested in neuron-glia interactions. Over the past 15 years Hong has studied the mechanisms of inflammation-related progressive neurodegeneration in Parkinson and other diseases. “We realized that non-neuronal cells, such as microglia and astroglia, play critical roles in the pathogenesis of neurodegeneration,” said Hong. He is trying to determine how microglia and astroglia communicate. Hong and other members of his lab join the SIG meetings remotely through videoconference.
“Microglia are quite interesting because they are dynamic,” said NINDS senior investigator Dorian McGavern, chief of the Viral Immunology and Intravital Imaging Section. McGavern uses two-photon microscopy to compare microglia biology during a state of viral persistence or traumatic brain injury. We are “seeing dynamics, not just a snapshot in time, but [four-dimensional] imaging,” said McGavern about the real-time tracking of microglia in a neuroprotective state after transcranial delivery of an antagonist. “Now we want to use an agonist to foster the ‘jellyfish’ reaction,” a term he recently coined in a Nature publication to describe activated phagocytic microglia (Nature 505:223–228, 2014).
Sohyun Ahn (NICHD) does not study glia directly, but instead investigates neural stem cells (NSCs), which express markers similar to those of glia and are even called radial glia during development. “We are interested in glial connections and the intrinsic NSC behavior,” she explained. Her goal is to manipulate endogenous NSCs to produce cell types needed in disease models for therapeutic purposes.
Karlyne Reilly (National Cancer Institute) is researching two rare, incurable nervous-system tumors that arise from glial cells: astrocytomas in the central nervous system and malignant peripheral nerve-sheath tumors in the peripheral nervous system. “We mostly focused previously on genetic background,” said Reilly. “We now want to look at mechanisms of tumor cells and the glial environment, specifically looking at the extracellular matrix.” She joined the NGI SIG in hopes of expanding her research by “interacting with people [who] know normal glial processes.”
NGI SIG meetings, held on the first Tuesdays of the month at 2:00 p.m., are open to members of the NIH community as well as researchers in the Washington, D.C., metropolitan area. The meetings feature presentations by outside and NIH speakers as well as by students and postdocs. For more information about the SIG, e-mail Amy Shafqat at amy.shafqat@nih.gov. To join the LISTSERV, send your request to neuron-glia@list.nih.gov.
This page was last updated on Wednesday, April 27, 2022
