AI-Powered ‘Digital Twins’ Predict Liver Donors’ Futures
IRP Study Shows Potential of Machine Learning Algorithms in Personalized Medicine

IRP researchers used artificial intelligence tools to design a computer program that could help guide the recovery process for people who donate a portion of their liver to a transplant patient.
Despite the potential drawbacks of time travel demonstrated in countless sci-fi movies, most people wouldn’t mind some advice from their future self. What they might not think about is how useful their doppelganger’s knowledge of the future could be to their doctor. IRP researchers hope an AI-powered computer model they’ve developed could provide those kinds of predictive medical insights for people recovering after donating a portion of their liver to someone in need of a transplant.1
More than 10,000 patients received liver transplants in 2023, a record-setting year for the procedure. A small number of them received liver tissue from a living donor thanks to the liver’s unique ability to regenerate.
“The liver is a very special organ,” notes IRP senior investigator Vipul Periwal, Ph.D., the new study’s senior author. “It’s been known since the time of the ancient Greeks that if you cut off a piece of a person’s liver, the rest of the liver will grow to have the same mass that it had before the surgery. This doesn’t even take a long time. If someone takes out 65 percent of your liver, it will actually regrow within about a year.”
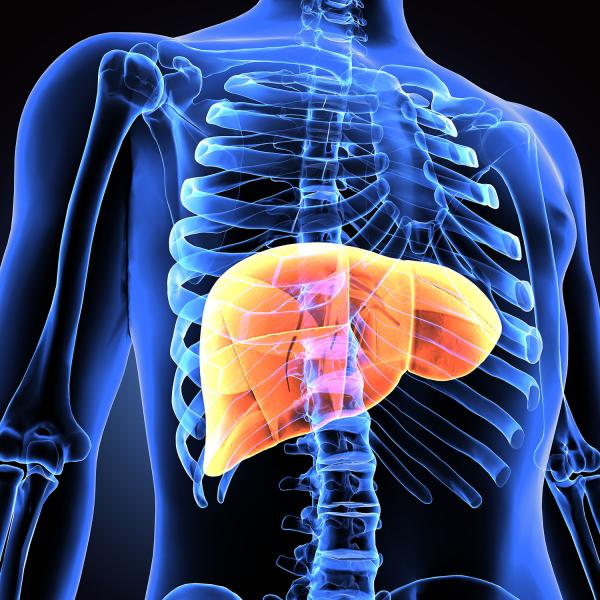
Unlike other internal organs, the liver can fully regenerate if a portion of it is removed.
However, that doesn’t mean recovery is always smooth sailing for those who donate a portion of their liver. Examining the activity of a donor’s genes, known as gene ‘expression,’ can potentially help out by telling a doctor what is going on in someone’s liver, but knowing what a patient’s genes are doing, say, three months after the surgery still leaves the question of what their gene expression should look like at that time if their liver is regenerating properly. Knowing the former but not the latter is like seeing that you’re driving on Route 66 without realizing you ought to be on the Blue Ridge Parkway.
That’s why Dr. Periwal and a group of researchers led by IRP postdoctoral fellow Suvankar Halder, Ph.D., the new study’s first author, turned to AI to try to predict what the ups and downs of a patient’s gene expression will be during the year after they donate a portion of their liver if the organ’s regeneration is on track. Doctors can then compare that predicted gene expression trajectory to the patient’s actual gene expression in the months after the procedure to identify and address any potentially problematic deviations.
“The physician only needs one pre-surgery blood sample to be able predict gene expression over the whole year of the recovery process,” explains Dr. Halder. “Then, if the physician sees something is higher or lower than it should be during the normal recovery process, they can intervene early.”
“You can’t keep doing abdominal imaging over and over to assess liver mass,” Dr. Periwal adds. “That requires a lot more staff and infrastructure than getting gene expression from a blood draw.”

The AI tool tells doctors how active various genes should be if a living liver donor’s recovery from the procedure is on track.
The research group created their predictive and personalized AI tool by providing a ‘machine learning’ computer algorithm developed in Dr. Periwal’s lab with data on thousands of genes’ expression levels in eight living liver transplant donors at 14 pre- and post-surgery time points. The computer program then used that data to teach itself to use a patient’s pre-surgery gene expression levels to predict how all those genes should behave over the year following the surgery if recovery is going well. Finally, the researchers tested their predictive program by feeding it gene expression data from one pre-surgery blood sample for each of four other patients without providing the algorithm access to any data from post-surgery blood samples. They found that the algorithm’s predictions about each of those patient’s individual gene expression during the year after surgery closely matched the patients’ actual gene expression during that period as their livers successfully regenerated.
“It’s all about being able to predict how a patient should be recovering on an ideal recovery track,” Dr. Periwal says. “If a patient starts to deviate from that expected recovery track, then you know you have to look closer at what is going on with that particular patient. You can see which genes are not doing what they should be, and because the genes have known functions, you can go back and ask, for instance, why the liver isn’t processing sugar like it should or is producing more fat than it should. Knowing what is going awry means you can start looking at the bigger picture. The liver is doing so many things, and if any of these things is going wrong, you want to know.”
In addition to making accurate predictions for real patients, the AI program also allowed the researchers to test out hypothetical scenarios by tweaking the initial gene expression data provided to the algorithm. In this way, they could learn about which genes play larger or smaller roles in the liver regeneration process and when during the recovery process those genes have the strongest influence. Such simulations could potentially help scientists create ways to assist with liver regeneration if needed by manipulating specific genes at specific times during recovery.

Dr. Vipul Periwal, the study’s senior author (left), and Dr. Suvankar Halder, the study’s first author (right)
“There are some genes you should pay more attention to because they have more of an effect,” Dr. Periwal says.
As useful as Dr. Periwal’s and Dr. Halder’s predictive AI tool could be for doctors treating patients who have donated part of their livers, Dr. Periwal also sees it as a powerful demonstration of how AI-powered machine learning algorithms could revolutionize personalized medicine in the future. He is particularly eager to create a similar predictive tool that can help doctors plan out and adjust the doses and types of chemotherapy they provide to cancer patients.
“That would be something I would really think would be an ideal application,” he says. “It’s very hard to figure out what the appropriate schedule for the treatments is, and it has to be individualized. There aren’t that many live donor liver transplants, so for us this is kind of a proof-of-concept for how you could have initial gene expression, put that into a computer model, and make gene expression predictions for later time points. We know how to do this now systematically, but the key is to apply it to processes where it could make a big impact on patients’ health. Every cancer is different, so I think that would be the ideal case where our work could make a big impact.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Halder S, Lawrence MC, Testa G, Periwal V. Donor-specific digital twin for living donor liver transplant recovery. Biol Methods Protoc. 2025 May 10;10(1):bpaf037. doi: 10.1093/biomethods/bpaf037.
Related Blog Posts
This page was last updated on Monday, August 18, 2025
