From Friend to Foe: When the Immune System Turns on the Brain
IRP Research is Exploring the Role of Immune Cells in Dementia
Scientists at NIH’s Center for Alzheimer’s and Related Dementias are examining how an immune system overreaction in the brain induces Alzheimer’s and other forms of dementia.
If you visit a lab at NIH’s Center for Alzheimer’s and Related Dementias (CARD), you may find yourself surrounded by several robots hard at work nurturing the hundreds of sets of genetically modified stem cells that CARD scientists use to study the illnesses that give CARD its name. Many of these cells will be coaxed to mature into the neurons that power our movements, thoughts, and memories — but not all of them. Neurons have long received the lion share of dementia researchers’ attention, understandable seeing as the visible symptoms of Alzheimer’s disease are closely linked to a build-up of proteins — amyloid-beta and misfolded tau — that damage neurons. However, neurons aren’t the only brain cells involved in dementia.
“There was a time when the human brain was thought to be 'immune privileged,' meaning the body’s immune cells did not get involved in it,” says IRP posbaccalaureate fellow Sydney Klaisner, who works with CARD’s Proteomics Expert Group. However, today we know that is not quite true. Together with her colleagues at CARD, Sydney is expanding the horizons of dementia research to investigate the key role the immune system seems to play in brain-destroying diseases.
The Brain’s Alarm System
When you think of your immune system, you might imagine an army of cells that protect you from outside threats like viruses and bacteria. However, the immune system also detects internal threats like the misbehaving proteins associated with Alzheimer’s disease.

Sydney works on preparing samples for analysis in the CARD lab.
Sydney and her CARD colleagues are specifically looking at how immune cells called microglia detect and react to abnormalities in the brain. Of the several types of immune cells found in the brain, microglia outnumber the rest by a significant margin. One of their jobs is to work as the brain’s clean-up crew by gobbling up clumps of abnormal proteins and damaged neurons. They can also alert the rest of the immune system about problems.
One way microglia do this is by using human leukocyte antigens (HLAs), proteins that end up on the surface of many cells in the body and act as a barcoding system. This system evolved as a sort of security alarm to protect us from pathogens like viruses that might otherwise hide from the immune system inside infected cells. HLA molecules start inside a cell, where they bind to chopped-up fragments of proteins, known as peptides. They then carry these peptides to the cell’s surface in order to present them to other immune cells. In microglia, HLA class I proteins carry peptides from proteins created by the microglia themselves, while HLA class II proteins carry peptides from proteins the microglia have gobbled up from around the brain.
Meanwhile, other immune cells, such as T-cells, act as security guards; they probe the surface of cells, including microglia, for any strange-looking peptides being presented to them by HLA. If a T-cell sees an abnormal peptide presented by HLA on the surface of microglia, it can alert the rest of the immune system and trigger a cascade of inflammation.
“In the past, many scientists believed that cells in the central nervous system, including microglia, lacked HLA class one molecules,” Sydney says. “Newer studies show that these HLA genes are highly active in microglia. Most of these studies have been performed in mice, but we are bringing these studies into human cells.”
CARD’s iPSC Neurodegenerative Disease Initiative (iNDI) project enables these studies. As part of the iNDI project, CARD’s Automated Cell Culture team has created models of Alzheimer’s disease in a dish, allowing researchers to follow changes in cellular processes triggered by specific genetic variants. These studies start with genetically engineered stem cells that can become any other cell type, and then CARD researchers coax these stem cells into neurons, microglia, and other types of brain cells for lab experiments.

Erika Lara Flores, lead of the Automated Cell Culture Expert Group at CARD, works on CARD’s cell culture robot.
Sydney and other researchers at CARD are using iNDI cells developed into microglia to investigate the role of the brain’s immune system in dementia. They’ve found evidence of HLA proteins on the surface of human stem cell-derived microglia. Using data science algorithms, they’ve also predicted that fragments from key mutated proteins associated with dementia may get presented by HLA on the surface of microglia.
An Immune System Backfire
Many home cooks have gotten distracted and left something on the stovetop for too long, burning their food or even causing a small fire in the pan. If they reacted quickly enough, their fire alarm might not have even gone off, or if it did, they most likely quickly disarmed it after dealing with the problem.
In contrast, Alzheimer’s disease and other dementias appear to occur when the brain’s own fire alarm won’t turn off once activated. A healthy immune system puts out fires early and efficiently and then goes to rest on the couch, but in those illnesses, microglia and other immune cells become chronically activated. Like a firefighting crew that breaks down the door and soaks an entire apartment to put out a small cooking fire, the brain’s immune cells can wreak havoc in the brain in an attempt to deal with abnormal proteins and protein buildup.
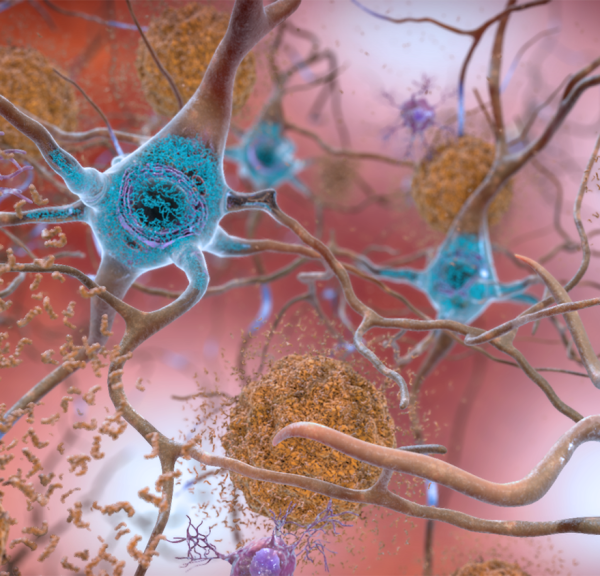
Amyloid-beta plaques (brown) and mutated tau (blue) are two of the main abnormal proteins that may trigger an immune response in the brain that contributes to dementia.
Researchers have found that many genes containing genetic variants associated with dementia actively make RNA and proteins in microglia. HLA molecules may carry mutated proteins created by these genes, such as mutated tau, to the surface of microglia, where they signal other immune cells that something is wrong. This means that microglia may not even have to detect and engulf neurons damaged by mutated tau inside them in order to set off the brain’s fire alarm. Like firefighters, an army of activated T-cells can then break down the door of the blood-brain barrier, inadvertently bringing with them neuron-killing inflammation.
“Before I started working at CARD, I didn’t realize how powerful the immune system in the brain can be, and that it may be detrimental in some cases,” Sydney says.
Does HLA Interact with Mutated Proteins?
Scientists at CARD and elsewhere are making it increasingly clear that microglia are involved not only in clearing damaged and dying neurons but also in actively promoting an immune response that could lead to further damage. When they detect protein changes in the brain, microglia may respond by over-pruning connections between neurons, killing brain cells, and triggering damaging inflammation. Since neurons cannot regenerate, that immune response can accelerate cognitive decline.
However, questions remain about how microglia monitor the brain for mutated proteins and which mutated proteins they react to. In her research, Sydney has been looking at which peptides within microglia can bind to HLA. She uses a technique called mass spectrometry to analyze all the HLA-bound proteins in samples of brain tissue and various types of cells generated using iNDI stem cells.

A mass spectrometer in the CARD lab. Mass spectrometry allows CARD scientists to identify all the peptides that HLA carries from within microglia to the surface of the cells.
“We use an ‘immunoprecipitation’ process that is long and complicated, but challenging and fun,” Sydney says. “We have these tiny beads with antibodies attached to them that interact with HLA. We are able to scrape just the surface of microglia to isolate the HLA-peptide units, and then break the interaction between HLA molecules and the peptides they have bound and brought to the surface. This allows us to see which proteins interact with HLA.”
Already, Sydney and her colleagues are seeing that non-mutated forms of proteins such as tau, APOE, and PARK7 interact with HLA, and that HLA molecules bring fragments of these proteins to the surface of microglia. These proteins are created by genes — MAPT, APOE, and PARK7, respectively — that commonly contain genetic variants linked to neuron-destroying diseases. If mutated versions of these proteins also interact with HLA and are presented to the microglial cell surface, T-cells will recognize them as foreign and begin their assault on the brain. This seems likely to be the case, at least for tau, as Sydney and her colleagues have found evidence using computer simulations that peptides derived from abnormal tau proteins strongly interact with certain forms of HLA.
“We are now planning to introduce synthetic mutated peptides into microglia to observe if they get processed and presented by HLA on the microglial surface,” Sydney says.
Immune Responses — Potential Therapeutic Targets?
Understanding how HLA contributes to inflammation in the brain and the eventual destruction of neurons may open up new therapeutic approaches. Sydney and others at CARD recently observed that when they introduced inflammatory molecules to microglia growing in a petri dish, the number of certain types of HLA on the surface of the microglia skyrocketed. This suggests that when the brain is in a state of inflammation — such as during Alzheimer’s disease — HLA activity may increase.
“Microglia play such a complicated role in brain health,” Sydney says. “We are finding some interesting things by diving into how microglia use HLA to recruit other immune cells in their fight against dementia-related brain changes. Most people I talk to are surprised to hear how relevant the immune system is to these diseases. I’m excited to publish our initial findings, hopefully soon.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
Related Blog Posts
This page was last updated on Monday, November 4, 2024
