Camel-Derived Therapy Infiltrates Cells to Treat Multiple Sclerosis
IRP Mouse Study Shows Promise of Treatment Strategy

A unique miniature antibody taken from camels shows promise for alleviating the symptoms of the devastating neurological disease multiple sclerosis, according to recent IRP research.
Earth’s jungles, deserts, and oceans are chock-full of wonderous creatures that have inspired a wide array of cutting-edge technologies, from strong yet flexible clothes made of synthetic spider silk to the plant-derived aspirin and morphine that have long been used as painkillers. Over the past few years, scientists at NIH and elsewhere have added sharks and camels to that list due to unique molecules their immune systems make. IRP researchers recently showed that one of those molecules could potentially be used to treat the devastating neurological disease known as multiple sclerosis.1
For many, news coverage of the COVID-19 pandemic provided a crash course on antibodies, the proteins our immune system makes to combat disease-causing pathogens like the novel coronavirus. Antibodies do this by binding to molecules on the surface of viruses, bacteria, or even disease-causing cells like tumor cells. However, sometimes the molecules that cause disease reside inside cells rather than on them, making them much more difficult to target.
Enter nanobodies, much smaller versions of antibodies that are only made in the bodies of a few species, including sharks and members of the biological family Camelidae like llamas and, of course, camels. Not only are nanobodies small enough to pass through the blood-brain barrier, making them useful as treatments for neurological diseases, but they are also small enough to enter our cells.
“The beauty of them is that nanobodies are similar to our own antibodies, so they don’t cause an immune reaction, and they are also very small and very specific,” explains IRP senior investigator Charles Egwuagu, Ph.D., the new study’s senior author.
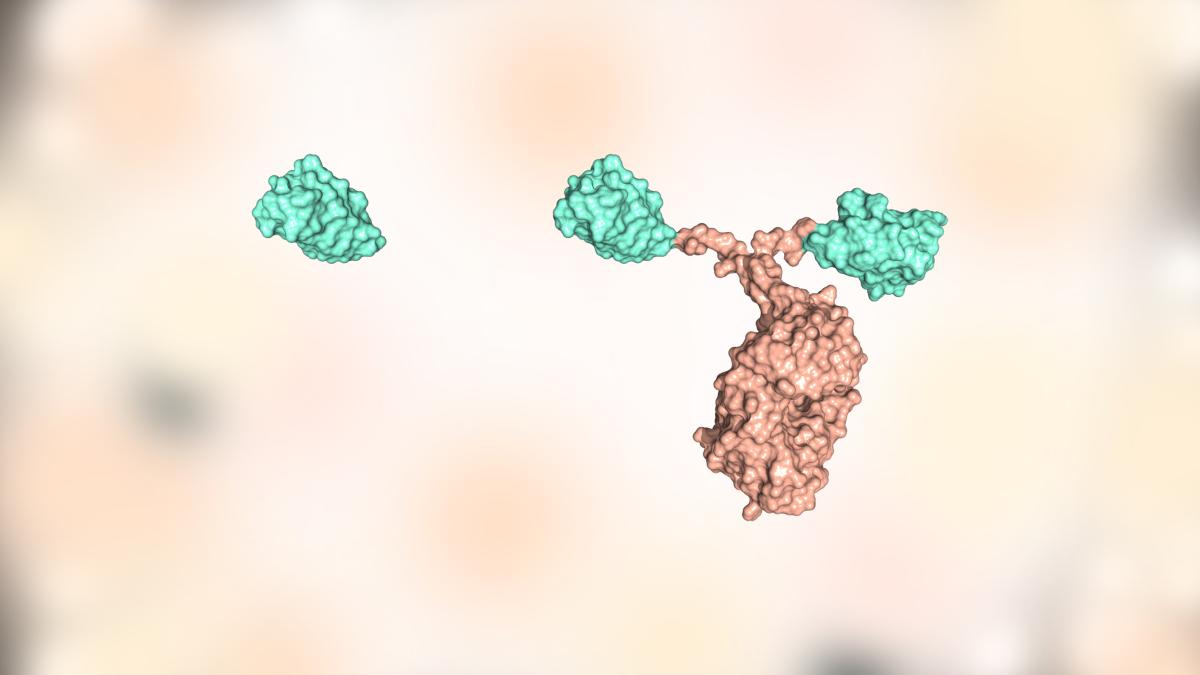
Unlike traditional antibodies used for medical purposes (right), nanobodies that come from sharks, llamas, or camels (left) are small enough to enter cells and alter their behavior from within.
For those reasons, Dr. Egwuagu has spent the past few years studying how nanobodies might be used to treat autoimmune conditions that occur when immune cells attack parts of the central nervous system. He has been particularly interested in nanobodies that target ‘transcription factors,’ molecules inside our cells that influence the activity of their genes. Since a cell’s genes direct how it behaves, inhibiting transcription factors could stop cells from acting in harmful ways.
A few years ago, Dr. Egwuagu’s lab and its collaborators used camels to create a nanobody called SBT-100 that can enter immune cells and inhibit a transcription factor called STAT3, a member of a family of transcription factors collectively known as STATs. The researchers then used the antibody to successfully treat mouse models of autoimmune uveitis, a blinding disease in which immune cells called Th17 cells attack the eye’s retina.2 The treatment worked because more generalized immune cells called T cells need STAT3 to develop into those more specialized Th17 cells.
However, it turned out that SBT-100 can also inhibit another member of the STAT family, called STAT1, which is essential for turning T cells into Th1 cells. Those Th1 cells, along with their Th17 cousins, just so happen to be the main drivers of multiple sclerosis, a neurological disease in which the immune system attacks the fatty material, called myelin, that surrounds neurons and helps them transmit messages. This leads to progressive paralysis and, in some cases, eventually death. Unfortunately, there is no cure for the disease and the few available treatments only slow its progression temporarily.
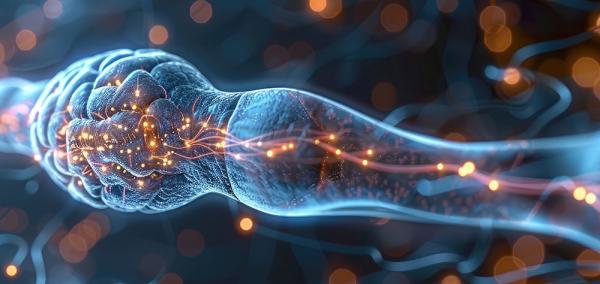
The signal-transmitting arms of neurons are wrapped in a fatty material called myelin, which helps them send electrical signals across the brain. Multiple sclerosis occurs when immune cells attack that myelin coating.
In the new study, Dr. Egwuagu’s team used its SBT-100 nanobody to treat a mouse model of multiple sclerosis. They found that mice treated with the nanobody had less severe symptoms and fewer immune cells running around causing damage inside their brains and spinal cords. Moreover, when isolated in petri dishes and exposed to a protein found in neurons’ myelin coating, T cells from the brains and spinal cords of the treated mice multiplied much less than T cells from untreated mice, suggesting that the treatment curbed the cells’ vendetta against myelin.
The researchers next homed in on the specific Th1 and Th17 cells that drive multiple sclerosis. The two cell types each have their own particular way of wreaking havoc: Th1 cells by producing a substance called IFN-gamma and Th17 cells with a chemical called IL-17. Treating mouse models of multiple sclerosis with the SBT-100 nanobody reduced the number of T cells in the animals’ brains and spinal cords that produced IFN-gamma and IL-17, suggesting that the treated animals had fewer disease-causing Th1 and Th17 cells. What’s more, the treated mice also had fewer T cells with high levels of the molecules that allow Th1 cells to make IFN-gamma and Th17 cells to produce IL-17.
“Th1 cells have their own signature molecule, which is Tbet, and Th17 cells’ signature molecule is ROR-gamma-T,” Dr. Egwuagu says. “If you do not have ROR-gamma-T, you don’t have Th17 cells, and if you don’t have Tbet, you don’t have Th1 cells.”
Finally, Dr. Egwuagu’s team tested what would happen when they took T cells from treated or untreated mice and transferred the cells into a different group of untreated mice. The researchers found that T cells taken from treated mice penetrated into the brains and spinal cords of their new, untreated hosts much less than T cells from untreated mice, and the cells also produced less IFN-gamma and IL-17. What’s more, mice that received T cells from treated counterparts had less severe symptoms than those that received T cells from untreated mice.

Dr. Charles Egwuagu
“By taking the T cells and only the T cells — nothing else — from the treated mice, you can show that these T cells have lost the ability to cause the disease,” Dr. Egwuagu says.
Collectively, the new study’s results suggest that the SBT-100 nanobody might make for an effective treatment for multiple sclerosis and, potentially, other diseases that result from an immune cell assault on the central nervous system. What’s more, because many tumor cells have high levels of STAT3, a nanobody like SBT-100 that suppresses STAT3 could eventually find uses in treating cancer as well. Rather than help move SBT-100 from the bench to the bedside, though, Dr. Egwuagu’s team is looking into other transcription factors that nanobodies from the Camelidae clan might be able to inhibit, which could lead to new treatments for a wide array of ailments.
“Some of the challenges in treating cancer and autoimmune diseases is that the things you want to target are inside cells, and many people don’t know how to get therapies inside the cell,” Dr. Egwuagu says. “What I want to do is see whether this nanobody approach could be a general way to target critical molecules inside cells that you usually can’t get at.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Mbanefo EC, Seifert A, Yadav MK, Yu CR, Nagarajan V, Parihar A, Singh S, Egwuagu CE. A Camelid-Derived STAT-Specific Nanobody Inhibits Neuroinflammation and Ameliorates Experimental Autoimmune Encephalomyelitis (EAE). Cells. 2024 Jun 16;13(12):1042. doi: 10.3390/cells13121042.
[2] Mbanefo EC, Yan M, Kang M, Alhakeem SA, Jittayasothorn Y, Yu CR, Parihar A, Singh S, Egwuagu CE. STAT3-Specific Single Domain Nanobody Inhibits Expansion of Pathogenic Th17 Responses and Suppresses Uveitis in Mice. Front Immunol. 2021 Sep 15;12:724609. doi: 10.3389/fimmu.2021.724609.
Related Blog Posts
This page was last updated on Tuesday, October 1, 2024
