Dialing Down the Brain’s Pain Thermostat
IRP Researchers Discover Center for Pain Control in the Brain

In recent years, IRP researchers have made important discoveries about how the brain ratchets up and tamps down the intensity of pain sensations.
While pain may be a sensation created by the brain, that doesn’t mean it’s all in your head. New research is showing how a delicate interplay between opposing types of neurons deep within the brain dials pain sensations up and down in response to injuries and other experiences.
September is Pain Awareness Month, a time to recognize that pain is a fact of life. However, while short-term pain is a critical warning system that keeps us from touching hot stoves and prompts us to visit the doctor for necessary medical care, the chronic pain experienced by nearly 100 million Americans often serves no protective purpose. To add insult to injury, this constant and often debilitating pain can evade both explanation and effective treatment.
“We know the brain influences how we perceive pain, but how it modulates it is still largely unknown,” says IRP investigator Yarimar Carrasquillo, Ph.D., whose lab is trying to solve this mystery in the hopes of improving diagnosis and treatment for chronic pain.
“We focus on a brain structure called the central amygdala, a tiny but mighty structure that has been shown to be involved in emotions and forming memories,” she continues. “I was involved in studies that showed that, additionally, the amygdala plays an important role in processing pain.”
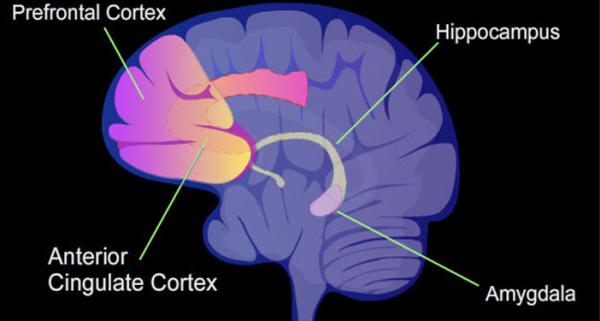
Dr. Carrasquillo’s research focuses on a brain structure called the amygdala, which is known to be involved in emotion and memory but has more recently been linked to pain processing.
Dr. Carrasquillo first became interested in the amygdala’s role in pain modulation while in graduate school. Research done in the 1980s and 1990s had shown that the amygdala could reduce the sensation of pain, but newer studies complicated the situation by suggesting the amygdala can also increase those sensations.
“For almost 20 years, I was bothered by that seemingly conflicting finding,” Dr. Carrasquillo explains, “so, when I started my lab at the NIH in 2014, that was the first question I really wanted to address.”
That opportunity, combined with significant advances in tools for manipulating the genes of specific cells and entire animals, allowed Dr. Carrasquillo and her colleagues to take a much closer look at the neurons within the amygdala. They discovered that there are two types of neurons involved in pain distinguished by different levels of activity in certain genes, which scientists refer to as gene ‘expression.’ This observation led Dr. Carrasquillo to theorize that perhaps one of these two sets of neurons was involved in increasing pain while the other had a role in decreasing it. And that’s exactly what further experiments revealed.
“It was so cool to actually see it after 20 years of reading these conflicting study results,” Dr. Carrasquillo says. “We were able to resolve the conflict by showing that, actually, everyone was right. The amygdala can both increase and decrease pain based on the activation of specific neurons.”
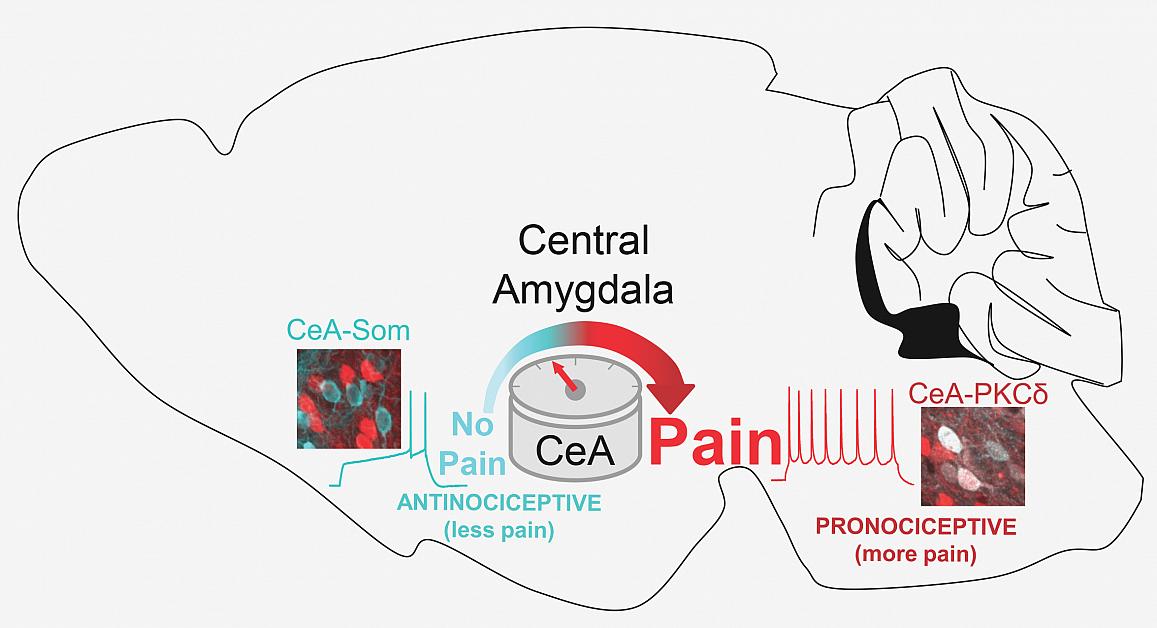
Dr. Carasquillo’s team discovered that amygdala neurons containing a substance called PKC-delta (red) increase pain sensations while those containing somatostatin (blue) suppress those sensations.
Dr. Carrasquillo's laboratory made their discovery by using a variety of laboratory techniques to determine which neurons were activated in a mouse model with a form of ‘neuropathic’ pain that resembles sciatica, a common nerve pain that shoots from hip to knee. Her team discovered that one type of neuron in the amygdala contains a substance called protein kinase C-delta (PKC-delta), and these cells are sensitized by injury to the nerve and subsequently increase pain responses. Other neurons in the amygdala that contain a substance called somatostatin are instead inhibited by nerve injury and act to reduce pain. The twin discoveries suggested that those two types of neurons function in opposition to each other, amplifying or suppressing pain based on the circumstances around an injury.1
Dr. Carrasquillo’s lab generated further support for that theory by leveraging a cutting-edge technology called chemogenetics, which allows scientists to specifically activate only one type of neuron in mice by injecting the animal with a certain drug. Dr. Carrasquillo and her colleagues used this approach to selectively turn each of the two types of neurons in the amygdala on or off and then observe the effects in the mouse model of sciatic pain. These experiments showed that inhibiting PKC-delta neurons in injured animals appears to relieve pain, while turning them on in healthy mice appears to cause pain. Conversely, suppressing the activity of the neurons containing somatostatin causes uninjured animals to exhibit signs of pain.
“Basically, from all of these different angles, we keep seeing the same thing,” Dr. Carrasquillo says: that PKC-delta neuron activity increases with injury and drives pain, whereas the somatostatin neurons go quiet during injury and tamp down pain when they are activated.

Dr. Yarimar Carrasquillo
In this sense, Dr. Carrasquillo explains, pain is “all in the brain,” by which she means that pain can be driven by changes in the nervous system, rather than a physical injury. For chronic pain patients with no observable problems that could cause their pain, this finding is particularly important. They also highlight the complexity of the pain experience.
“There doesn’t need to be an injury to experience pain,” Dr. Carrasquillo says. “Perhaps the problem is in the nervous system. They’re not making it up.”
“Pain needs attention from multiple levels,” she adds. “Just injecting something to numb a patient’s injury or decrease inflammation may not be enough because there are so many things happening in the nervous system. The hope in the long term is that our findings will help us develop better treatments and better diagnostic tools, especially for patients who have trouble getting anyone to believe they are suffering.”
References:
[1] Wilson TD, Valdivia S, Khan A, Ahn HS, Adke AP, Martinez Gonzalez S, Sugimura YK, Carrasquillo Y. Dual and Opposing Functions of the Central Amygdala in the Modulation of Pain. Cell Rep. 2019 Oct 8;29(2):332-346.e5. doi: 10.1016/j.celrep.2019.09.011.
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
Related Blog Posts
This page was last updated on Wednesday, September 6, 2023
