Postdoc Profile: A Bird’s-Eye View of Retinal Disease
Dr. Noor White Traces Evolution to Identify Genes Critical for Vision

Dr. Noor White
The eye has existed in some form for roughly 600 million years. Its many intricate components and the general ability of organisms to sense light have continued to adapt and evolve over huge spans of time into what we know as vision today. By mapping out the evolution of vision, Noor White, Ph.D., hopes to shed light on the genetic causes of diseases that affect the retina, the part of the eye that turns light into electrical signals the brain can use to build an image of our surroundings.
“If we can take a step back and look at the bigger picture, then we can identify the critical genetic components of vision,” explains Dr. White, who was an IRP postdoctoral fellow for four years before becoming a Staff Scientist in March.
Dr. White’s interest in vision started with birds. Birds have a particularly wide variety of visual abilities: some see ultraviolet light, some prefer the color blue, some can see underwater or from incredible distances — and, of course, some birds can see at night.
When Dr. White joined the National Eye Institute (NEI) in 2018, she planned to study the evolutionary history of nocturnality and night vision in birds. However, alongside IRP senior investigator Anand Swaroop, Ph.D., she has expanded her research to examining vision in all animals with a backbone, known as vertebrates. Together with colleagues in her lab, she has begun to identify which genes are critical to vision by tracing how vision has evolved over time.
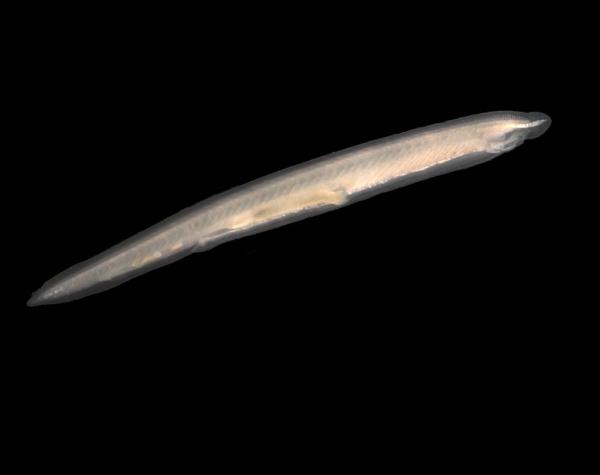
Image credit: Hans Hillewaert via Wikimedia commons
Dr. White discovered that primitive organisms called lancelets, pictured here, may possess an early version of the NRL gene.
Dr. White began by creating a large dataset of genetic material from over 100 species, and she is now using it to narrow down when in evolutionary history vertebrate vision first appeared. She has specifically focused on identifying the emergence of rod cells, light-sensing cells in the retina that help us see in low-light conditions. In humans, the NRL gene has been identified as key to rod development, and using her database, Dr. White has discovered a gene that may be an evolutionarily early form of NRL in fish-like organisms called lancelets. Lancelets possess primitive versions of many of the organ systems found in humans and other vertebrates, making them a common focus for evolutionary biologists like Dr. White.
To confirm whether this ancient version of the NRL gene marks when rods first developed, Dr. White has inserted it into the retinas of newborn mice lacking the NRL gene and is waiting to see if these mice develop rod cells. If they do, it will indicate that this early genetic code is sufficient for the development of rods in the eye. Once that is established, Dr. White can begin to look at other species and document genetic differences that appear to enhance rod development or diminish it. Future research could then begin to develop treatments based on what she discovers.
“By determining what genes support rod function, we will be able to determine what areas of the genome may be relevant for retinal disease,” Dr. White says.

Dr. White in the collections of the Smithsonian National Museum of Natural History, holding a potoo (left) and a hummingbird (right). These two birds were, respectively, the largest and smallest of the bird species she studied during her Ph.D. research.
That work involves examining the genomes of patients who are suffering from diseases that affect the retina and determining how their NRL genes differ from the evolutionarily early NRL gene Dr. White identified. Any differences in this part of the genome could be causes or predictors of disease. Already, Dr. White has narrowed down the list of genes that may be involved in the development of retinal disease.
She credits this initial success to her decision to study vision through an evolutionary lens, as opposed to via traditional cross-species comparisons, which she says allows her to “move beyond comparing and start to ask directional questions.” In other words, through knowing not only which species developed vision but also when they did so, she and her colleagues can more easily identify the genetic code critical to its development. Dr. White also acknowledges the importance of her arrival at NEI, which she views as a turning point in her research career.
“My research took off exponentially once I stepped foot on the NIH campus,” she says. “I wanted to work at NIH because I wanted to apply my skills and my efforts to something that would improve human health. I saw an opportunity for an evolutionary perspective to be put to practical use.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
Related Blog Posts
This page was last updated on Wednesday, July 5, 2023
