Designer Drug Uses Double Whammy to Fight Heart Disease
Custom-Built Molecule May Improve On Its Natural Counterpart
A synthetic drug developed at the NIH could provide a more effective way to lower levels of fat molecules in the blood in order to combat heart disease and other conditions.
Ten years ago, a young woman from Chicago came to the National Institutes of Health with a rare genetic condition. A mutation in her DNA was making her metabolic system malfunction, causing levels of fat molecules called triglycerides in her blood to skyrocket far out of the normal range. This triggered inflammation in her pancreas, a painful and potentially life-threatening condition known as pancreatitis. She couldn’t understand why there wasn’t any kind of treatment to help her.
IRP senior investigator Alan T. Remaley, M.D., Ph.D., took on the challenge with the help of Anna Wolska, Ph.D., a research fellow in his lab. Dr. Remaley leads the Lipoprotein Metabolism Section in the National Heart, Lung, and Blood Institute (NHLBI), where he and Dr. Wolska study lipoproteins, small particles that transport fats such as cholesterol and triglycerides through the bloodstream to be broken down and used by cells for energy. Their efforts to help that young woman ultimately led to the discovery — published last January — of a new strategy for reducing triglycerides in order to treat serious ailments like pancreatitis and heart disease.1
While the fats packaged inside lipoproteins are necessary for survival, having too many of them in the bloodstream is harmful. Numerous studies have linked increased triglycerides in the blood to a higher risk of heart disease, which affects about 125 million American adults, and acute pancreatitis, which causes around 275,000 hospital stays per year in the U.S. and appears to be on the rise. Drugs called statins can lower cholesterol levels by about 20 to 30 percent, and newer drugs may do even better, “but they typically do not have a large effect in lowering triglycerides,” says Dr. Remaley. Drugs that more effectively reduce triglycerides could help both people with pancreatitis and possibly, when paired with statins, the even greater number of people with heart disease.

Dr. Alan T. Remaley (left) and Dr. Anna Wolska (right)
Triglycerides are broken down into fatty acids by an enzyme called lipoprotein lipase (LPL), which is regulated by specific proteins. One of these proteins, called apolipoprotein C-II (apoC-II), activates LPL, triggering it to break down triglycerides. At the same time, a second protein called apolipoprotein C-III (apoC-III) blocks the action of LPL. Together the two proteins attempt to maintain a healthy balance of triglycerides in the bloodstream.
Factors such as diet, alcohol use, some medications, and genetic predisposition can disrupt this delicate balance and increase triglyceride levels. In rare instances, such as that of Dr. Remaley’s patient, people may have a serious genetic defect that significantly disrupts the system, leading to extremely high triglyceride levels. In that patient’s case, a mutation damaged apoC-II, allowing her triglycerides to accumulate to dangerous levels and thereby triggering her pancreatitis.
“Usually if you're a healthy person, you should have below 150 milligrams per deciliter of triglycerides in your blood,” explains Dr. Wolska. However, among people with a serious genetic defect, she says, “we may see over 10,000 milligrams per deciliter of triglycerides — their blood is basically cream.”
More From the IRP
Blog
Modified Hormone Protects Damaged Hearts
Because the absence of apoC-II was causing their patient’s problems, Dr. Remaley and Dr. Wolska thought perhaps they could create a synthetic peptide — essentially a short protein — to replace it. They began by enlisting the assistance of IRP senior investigator Richard Pastor, Ph.D., and his postdoctoral fellow Mohsen Pourmousa, Ph.D., both experts in computer simulations. Using a supercomputer located at the Pittsburgh Supercomputing Center in Pennsylvania, the pair of computer whizzes helped Dr. Remaley and Dr. Wolska simulate the movement of apoC-II as it interacts with fat molecules.
“That gave us a clue as to what piece of apoC-II we would need to synthesize so that our peptide would work,” says Dr. Remaley.
Next, the IRP team used that information to simulate how various configurations of many different peptide molecules would interact with LPL. In the end, the scientists identified several candidates with structures that could mimic apoC-II. They tested these on mice and found one, called D6PV, that worked remarkably well, lowering triglyceride levels by about 90 percent.
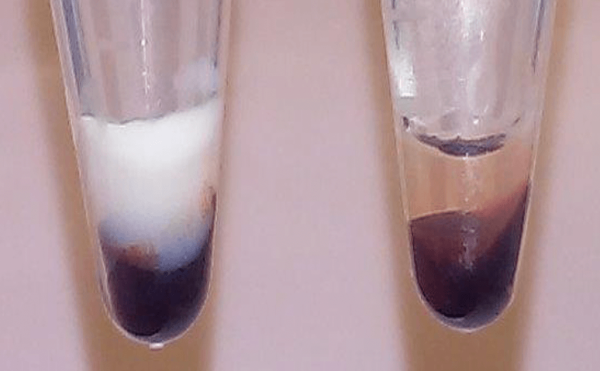
Much more milky, white fat can be seen in the blood of a mouse before treatment with D6PV (left) compared to after treatment (right).
“In an hour, you could see the effect of the peptide clearing all this fat from the blood,” recalls Dr. Wolska.
This in and of itself was an exciting discovery, but further tests showed that D6PV offered another important benefit. The surprising finding emerged when the scientists tested the designer peptide on mice bred to have very high triglycerides despite having functioning apoC-II.
“These mice are very sick from having lots of fat in the blood, but they do have apoC-II,” says Dr. Wolska. “Therefore, they wouldn’t necessarily benefit from getting the peptide, but we found that, actually, it worked efficiently on this mouse model too.”
This observation indicated that D6PV was lowering triglycerides through a second mechanism separate from its effects on apoC-II. It turned out that the designer peptide also blocked apoC-III, the protein that stops LPL from breaking down triglycerides.
“We sort of killed two birds with one stone,” says Dr. Remaley. “That was unexpected and the reason the finding was so exciting.”
In fact, several pharmaceutical companies are trying to develop something that can block apoC-III, and D6PV may fit the bill. One of them is Corvidia Therapeutics., based in Waltham, Massachusetts. The company, which was acquired by Novo Nordisk in June, entered into a Cooperative Research and Development Agreement (CRADA) with the NIH, a type of contract that makes government research, intellectual property, and expertise available to outside businesses so that they can quickly turn important discoveries made in the IRP into actual products that improve human health. Dr. Remaley and Dr. Wolska subsequently began collaborating with Corvidia to turn D6PV into a treatment for people with high triglycerides. Since striking the agreement, the IRP team, in collaboration with Corvidia scientists, have conducted additional experiments that show the peptide has an unusually long half-life, indicating it would last in the bloodstream for several days.
“We're hopeful that Novo Nordisk will carry it forward to make it into a drug,” says Dr. Remaley.
This video provides an overview of how D6PV works and why it is a promising treatment for patients with dangerously high triglyceride levels.
Ideally, Dr. Remaley hopes the company will eventually figure out a way to make an oral form of the drug. Barring that, D6PV’s long persistence in the body means it could be administered via an injection beneath the skin just once a week to dramatically lower levels of triglycerides in the blood and decrease the risk for heart disease and pancreatitis.
As for the woman from Chicago, Dr. Remaley hopes to bring her back to the NIH for treatment someday so she might benefit from the study she helped instigate.
“In fact, we sent the paper to her to let her know that she was the inspiration,” he says.
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References
[1] A dual apolipoprotein C-II mimetic-apolipoprotein C-III antagonist peptide lowers plasma triglycerides. Wolska A, Lo L, Sviridov DO, Pourmousa M, Pryor M, Ghosh SS, Kakkar R, Davidson M, Wilson S, Pastor RW, Goldberg IJ, Basu D, Drake SK, Cougnoux A, Wu MJ, Neher SB, Freeman LA, Tang J, Amar M, Devalaraja M, Remaley AT. Sci. Transl. Med. 2020 Jan 29; 12(528):eaaw7905. doi: 10.1126/scitranslmed.aaw7905.
Related Blog Posts
This page was last updated on Tuesday, May 23, 2023
