IRP’s Julie Segre Elected to the National Academy of Medicine
NIH Researcher Recognized for Investigation into the Skin Microbiome

IRP senior investigator Julie Segre was elected to the National Academy of Medicine in 2019 in recognition of her pioneering research into microbial genomics.
The National Academy of Medicine (NAM), first established in 1970 by the National Academy of Sciences as the Institute of Medicine (IOM), is comprised of more than 2,000 elected members from around the world who provide scientific and policy guidance on important matters relating to human health. Election to the NAM is considered one of the highest honors in the fields of health and medicine that recognizes individuals who have not only made critical scientific discoveries but have also demonstrated a laudable commitment to public service.
IRP senior investigator Julie Segre, Ph.D., was one of four IRP researchers elected to the NAM in 2019. Dr. Segre leads the Translational and Functional Genomics Branch at the National Human Genome Research Institute (NHGRI), where she studies the way in which the skin forms a barrier between the body and the environment. In particular, her research uses genetic sequencing to understand the bacterial and fungal microbes that live on human skin.
“Early on, science provided a framework for understanding the natural world, and genetics is part of the underlying code,” says Dr. Segre, whose grandfather served in military intelligence in World War II and taught her code breaking as a child. “Obviously, it then gets layered with life experiences, but those are the kinds of puzzles I like to work on.”
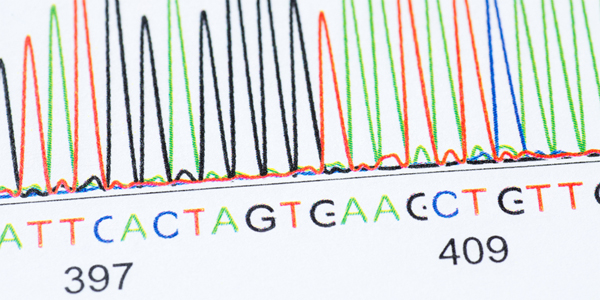
Advances in genetic sequencing have been crucial to Dr. Segre’s research on the skin microbiome.
The benign and disease-causing microbes on our skin form a microbial community called the skin microbiome. The study of the human microbiome, and particularly that of the skin, is a relatively new field catalyzed by advances in fast and affordable genomic sequencing along with a societal acceptance that bacteria, fungi, and viruses live on and around all of us. With that has come an appreciation that not all microbes are bad.
“The concept of a healthy microbiome is something that has really only developed in the past ten years,” says Dr. Segre. “For the microbes that live on our skin, one of their critical roles is to prevent disease-causing bacteria from infecting us.”
After studying mathematics as an undergraduate at Amherst College, Dr. Segre dove deep into genomics as a doctoral student at the Massachusetts Institute of Technology Genome Center, followed by a post-doctoral fellowship in skin biology at the University of Chicago. Her experiments in Chicago showed breaking the skin barrier stimulates production of antimicrobial proteins, which got her thinking about how our genes are influenced by our environment.
“I started to think about the microbes on humans — they are in some ways part of our genetic state, but they can be altered by environmental conditions and by lifestyle,” Dr. Segre explains. “They really sit at an important interface between gene-environment interactions.”
As chief of NHGRI’s Translational and Functional Genomics Branch and head of the Microbial Genetics Section, Dr. Segre has spent nearly 20 years studying and classifying the diverse bacterial and fungal microbes that populate human skin in both healthy individuals and those with skin disorders such as eczema. Working in close collaboration with the NIH Intramural Sequencing Center and the clinical departments of Infection Control, Microbiology, and Dermatology at the NIH Clinical Center, Dr. Segre’s group performs foundational studies to better understand the human skin microbiome and apply that knowledge to understanding how changes in microbial diversity are related to disease flare-ups, leading to new avenues to pursue for diagnosis and future therapies. For example, the topical treatments currently used to treat skin disorders may not only be restoring the skin’s barrier but also replenishing our skin’s microbial garden.
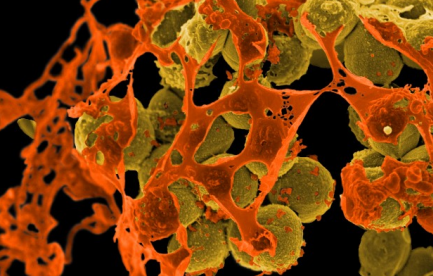
Overuse of antibiotics has given rise to antibiotic-resistant strains of bacteria such as methicillin-resistant Staphylococcus aureus, or MRSA (brown), which can cause serious skin infections.
Dr. Segre’s work could have particularly important implications for the use of antibiotics, which are currently used to treat many skin conditions despite widespread concerns in the medical field about bacteria developing resistance to such medications. The information her team is learning could result in ways to use the microbiome to fight off disease without antibiotics, such as skin creams that either deliver live microbes or promote growth of good bacteria that can outcompete disease-causing organisms.
“Antibiotics are very useful,” notes Dr. Segre, “but we want to use them wisely. We need more science to understand how we could do that, and that forms the basis for my future research.”
Understanding the genetic signature of these microbes is also changing the way we understand how infections are transmitted. Dr. Segre and her colleagues have pioneered ways to track infection transmission in hospitals by sampling surfaces and tracing the microbes’ genetic footprints. Her methods are becoming standard practice, especially in hospitals that care for more fragile patients.
“There’s a lot more emphasis on tracking hospital transmissions as we realize that we're running out of effective antibiotics,” says Dr. Segre.
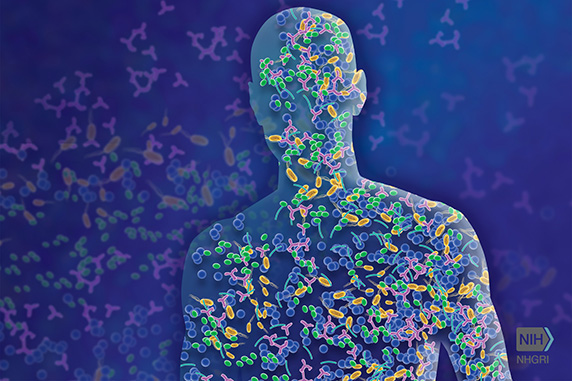
Thanks to Dr. Segre and her current and former members of her IRP team, scientists now know much more about the community of microbes living on our skin.
Dr. Segre is quick to share credit for her election to the NAM. After she found out about it, the first thing she did was send “letters of real appreciation” to the scientists who had mentored her in the past, as well as the three scientific directors she had worked under at the NIH. She then wrote similar letters to current and former members of her lab, noting the work they had done and “how much each of them had contributed to the success of the group.”
In addition to her scientific work, it is the evolution of this group that she is most proud of. Over the years, its members have expanded their knowledge and skills while taking off in new directions to explore subjects like wound healing and infection control.
“The NIH has been a wonderful combination of working with permanent employees who have worked in the group for ten-plus years, and the trainees who come through as students and postdocs,” says Dr. Segre. “We're very enthusiastic about being in on the ground floor of a new field. It's been very rewarding to have a diverse group of trainees because this field really needs diversity of experience and intellectual interests. There are so many new ideas that we can think about through this lens and come up with new understanding and new directions for research.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
Related Blog Posts
This page was last updated on Wednesday, July 5, 2023
