Find and Replace: DNA Editing Tool Shows Gene Therapy Promise
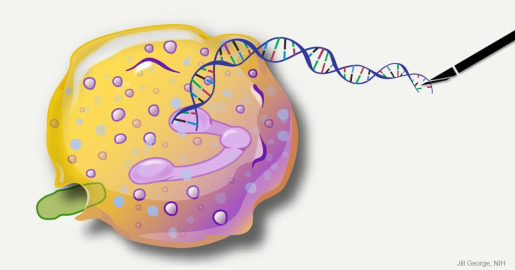
This image represents an infection-fighting cell called a neutrophil. In this artist’s rendering, the cell’s DNA is being “edited” to help restore its ability to fight bacterial invaders.
For gene therapy research, the perennial challenge has been devising a reliable way to insert safely a working copy of a gene into relevant cells that can take over for a faulty one. But with the recent discovery of powerful gene editing tools, the landscape of opportunity is starting to change. Instead of threading the needle through the cell membrane with a bulky gene, researchers are starting to design ways to apply these tools in the nucleus—to edit out the disease-causing error in a gene and allow it to work correctly.
While the research is just getting under way, progress is already being made for a rare inherited immunodeficiency called chronic granulomatous disease (CGD). As published recently in Science Translational Medicine, a team of NIH researchers has shown with the help of the latest CRISPR/Cas9 gene-editing tools, they can correct a mutation in human blood-forming adult stem cells that triggers a common form of CGD. What’s more, they can do it without introducing any new and potentially disease-causing errors to the surrounding DNA sequence [1].
When those edited human cells were transplanted into mice, the cells correctly took up residence in the bone marrow and began producing fully functional white blood cells. The corrected cells persisted in the animal’s bone marrow and bloodstream for up to five months, providing proof of principle that this lifelong genetic condition and others like it could one day be cured without the risks and limitations of our current treatments.
People with CGD carry one of several genetic mutations that leave their white blood cells unable to attack and kill infectious invaders. The glitches occur in genes encoding an important enzyme complex responsible for the blood cells’ normal antimicrobial activity. As a result, CGD patients must take special precautions to protect themselves, including a steady course of antimicrobial medications. Even then, they’re still at risk for developing life-threatening bacterial and fungal infections.
In the new study, scientists at the NIH’s National Institute for Allergy and Infectious Diseases (NIAID), including Harry Malech and Suk See De Ravin, set out to test the potential of CRISPR/Cas9 to help people with CGD. Using adult stem cells drawn from two patients with the same common CGD-causing mutation, the team tested in the laboratory the ability of CRISPR/Cas9 to fix the mutation. In this case, it was a single typo in the gene sequence on the X chromosome.
CRISPR/Cas9 uses small “guide RNA” molecules together with a scissor-like enzyme to find and snip the specific, incorrect DNA sequence in just the right spot. Once the DNA is cut, the cell completes the edit using the correct gene sequence from a DNA fragment supplied by the researchers as its template. Think of it as “find and replace” for the genome.
The researchers determined the CRISPR/Cas9 tools correctly repaired about 20 to 30 percent of the stem cells in a test tube. When it worked, the gene-editing technique replaced the faulty DNA sequence only. That’s because the guide RNA was truly specific enough to find and replace just the DNA sequence containing the single-letter typo.
That led to the next big test in which the researchers infused some 500,000 of the gene-edited cells into each mouse. Because the mice were immune deficient and had been pre-treated with the drug busulfan to suppress their own blood forming cells and “make room” for the transplant, their bodies accepted the human cells and allowed them to bring their immune systems into working order.
Sure enough, the infusions worked.The edited blood-forming stem cells settled into the bone marrow and produced mature blood cells, including the normal white cells called neutrophils that are lacking in people with CGD. After five months, about 10 to 20 percent of the blood cells still carried the correction. That’s notable because lasting correction of the gene in about 10 percent of blood cells would likely be enough to benefit patients.
While the findings are extremely hopeful, there’s much to do before the approach can be tested in people with CGD. The researchers say they’d like to get the editing right in an even greater proportion of stem cells. The process will also have to be scaled up, making it possible to correct many more cells. That’s because it will take hundreds of millions of edited cells to treat a much-larger human patient, not the half a million delivered to each mouse. In addition to their ongoing work in the lab, Malech and De Ravin are now exploring what’s needed to produce medical grade CRISPR/Cas9 tools suitable for use in the clinic.
While CRISPR approaches come with tremendous potential advantages in precision, other gene therapy approaches continue to show promise too. In fact, a clinical trial under way at NIH and other sites around the country is now testing a more-traditional approach to CGD gene therapy [2]. It uses an inactivated, non-infectious virus to insert a working gene into the cells of CGD patients. It’s too soon to know how well it will ultimately work, but early indications are encouraging.
The NIAID researchers have been working for decades to better understand CGD and find safe and more effective ways to treat this chronic and debilitating condition. This latest news is yet another encouraging sign of progress in treating CGD and many other inherited conditions, and it is a story well worth following in the years ahead.
References:
[1] CRISPR-Cas9 gene repair of hematopoietic stem cells from patients with X-linked chronic granulomatous disease. De Ravin SS, Li L, Wu X, Choi U, Allen C, Koontz S, Lee J, Theobald-Whiting N, Chu J, Garofalo M, Sweeney C, Kardava L, Moir S, Viley A, Natarajan P, Su L, Kuhns D, Zarember KA, Peshwa MV, Malech HL. Sci Transl Med. 2017 Jan 11;9(372).
[2] Study of Gene Therapy Using a Lentiviral Vector to Treat X-linked Chronic Granulomatous Disease. Clinicaltrials.gov
Links:
- Chronic Granulomatous Disease (CGD) (National Institute of Allergy and Infectious Diseases/NIH)
- Malech Lab (National Institute of Allergy and Infectious Diseases/NIH)
NIH Support: National Institute of Allergy and Infectious Diseases
Related Blog Posts
- RNA-Targeting Therapeutic Restores Protein Absent in Spinal Muscular Atrophy
- Innovation Awards Spark New Intramural Collaborations
- IRP’s Eugene Koonin Elected to National Academy of Medicine
- Mosquitos With Human Gene Hinder Malaria Transmission
- Tiny Molecules Have Big Potential for Treating Eye Diseases
This page was last updated on Wednesday, January 31, 2024
