Elucidating Brain Structure and Architecture
Peter Basser: Inventor of Diffusion Tensor Magnetic-Resonance Imaging
Advanced imaging technologies have revolutionized the fields of neuroscience and neurosurgery by revealing the complex architecture of the human brain. Two of those technologies—diffusion tensor magnetic-resonance imaging (DTI) and DTI streamline tractography—were invented at NIH in the 1990s by National Academy of Engineering member Peter Basser when he was in the Biomedical Engineering and Instrumentation Program (BEIP, the forerunner of the National Institute of Biomedical Imaging and Bioengineering, NIBIB). Today, neurosurgeons everywhere use planning software informed by DTI data to highlight critical brain regions that need to be avoided when excising tumors and performing other brain procedures. Basser holds several patents related to DTI technology which his lab uses to noninvasively study the microstructure of living tissues.

CREDIT: NICHD
Peter Basser, Ph.D.
Basser, who’s now a senior investigator at the Eunice Kennedy Shriver National Institute of Child Health and Human Development, described his inventions recently at the 15th annual Philip S. Chen Jr. Distinguished Lecture on Innovation and Technology Transfer in a presentation titled “Using Water Migration to Probe Brain Structure and Architecture.”
Diffusion is a mechanism by which water molecules move or migrate throughout tissue in response to thermal energy. That water migration can be detected with a magnetic-resonance imaging (MRI) technology called diffusion imaging. One complication is that tissue-water-diffusion properties are influenced by the fiber architecture, which was not adequately described by diffusion imaging methods. Basser and his colleagues developed and applied a new mathematical model to measure and map diffusion properties to characterize the microstructure of fibrous tissues, such as brain white matter.
It was at the 1991 NIH Research Festival that Basser stumbled across a poster presented by French physician Philippe Douek (a medical resident at NIH at the time) and his colleagues. The poster displayed MRI images of different parts of the brain, color-coded according to what the authors thought was the preferred orientation of diffusion in white-matter pathways. Basser had a eureka moment—there was a proper way to analyze those images. Previous methods had analyzed MRI data using mathematical constructs known as scalar diffusivities. He believed that using a different type of model known as a diffusion tensor could more accurately capture how molecules moved throughout tissue in specific directions, a property referred to as diffusion anisotropy. Using such a model would also allow for complex 3D mapping of axons in the brain, a technique that would later become known as streamline tractography. However, there was a problem: No one had ever measured a diffusion tensor before.
Basser painstakingly combed the literature. He was drawn to earlier work by John Tanner and Edward Stejskal, a pair of scientists at the University of Wisconsin-Madison (Madison, Wisconsin). They had first proposed the idea of using pulsed-field magnetic gradients to measure molecular diffusion and had theorized formulas to measure a tensor. In 1991, Basser began collaborating with James Mattiello at BEIP and Denis Le Bihan at the NIH Clinical Center. Basser and Mattiello became the first to measure a diffusion tensor within water and then later in a pork loin specimen.
They presented their novel method at the International Society for Magnetic Resonance in Medicine conference in 1992. According to Basser only about five people visited his poster, but in his Chen lecture, he emphasized the importance of persistence. In 1993, the group demonstrated diffusion tensor MRI, giving rise to DTI.
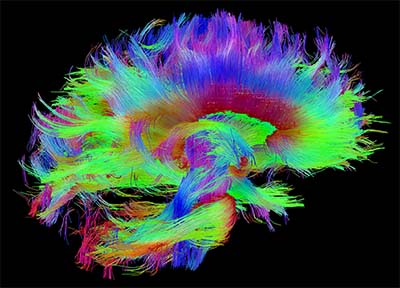
CREDIT: CONSORTIUM OF THE HUMAN CONNECTOME PROJECT
Some of Basser’s work on DTI tractography has inspired the creation of the Human Connectome Project and the NIH BRAIN initiative. This image, taken with diffusion spectrum imaging (a more advanced MRI technique), shows the architecture of white matter fibers in the human brain within the corpus callosum—a thick bundle of more than 200 million nerve fibers that connect the two hemispheres of the brain—and brainstem pathways. The fibers are color coded according to their anatomical orientation in the brain.
Basser went on to propose a family of tensor-derived quantities that have become widely used quantitative-imaging biomarkers. Throughout the 1990s, NIBIB’s Senior Investigator Carlo Pierpaoli and Basser worked together to define diffusion properties throughout the brain and bring the new technology to the clinic. In 1999, Pierpaoli and Sinisa Pajevic at NIH’s Center for Information Technology would also pioneer direction-encoded color (DEC) maps. DEC superimposes color on tissue to generate vivid maps of its structure and orientation. Today, clinical applications of DTI include stroke visualization, brain tumor assessment, and even the study of neuropsychiatric disorders by examining how the brain is wired.
DTI data have been critical to the inspiration of the NIH-supported Human Connectome Project, which provides an unparalleled compilation of neural data, and NIH’s BRAIN Initiative, which aims to revolutionize our understanding of the human brain. The DTI technology has even made its way into popular culture as seen in the science-fiction book Cerebranauts: In Search of the Human Experience by Seth Sherman and on t-shirts depicting brain maps.
As for what has yet to be discovered using DTI technology, Basser thinks we are just at the tip of the iceberg. “Spinal cord, peripheral nerves, cardiac muscle [are] just becoming imageable with DTI,” he said. “And there are a lot of possibilities for whole-body imaging that I think still can be and will be explored.”
The annual Philip S. Chen Jr. Distinguished Lecture on Innovation and Technology Transfer Lecture, which has been on hiatus for the past two years, was established in 2006 by the NIH deputy director for intramural research and the NIH Office of Technology Transfer (OTT) on the occasion of Chen’s retirement after more than 40 years of service to the NIH. Chen established the OTT to implement the provisions of the Federal Technology Transfer Act. He formulated the guiding principles upon which technology transfer functions today, including the creation of the Cooperative Research and Development Agreement, known as the CRADA. To watch a videocast of Peter Basser’s presentation, delivered on April 29, 2022, go to https://videocast.nih.gov/watch=44974.

Raghuram Reddy was a postbaccalaureate fellow in Desmond Brown’s lab in the National Institute of Neurological Disorders and Stroke, where he helped analyze the role of primary cilia in Parkinson disease and glioblastoma. In June 2022, he left NIH to attend medical school at Florida International University (Miami). Outside of work, he enjoys playing sports, taking photos, and hiking.
This page was last updated on Thursday, June 30, 2022
