Research Briefs
Read about NIH scientific advances and discoveries by intramural scientists: how sound reduces pain; bacterial populations concentrate in lung cancer cells; novel brain mechanism influences impulsive cocaine-seeking in rats; loss of youth protein may drive aging in the eye; scientists solve first-ever 3D structure of twinkle protein; monoclonal antibody prevents malaria.
NIDCR: YOUR BRAIN ON MUSIC; HOW SOUND REDUCES PAIN
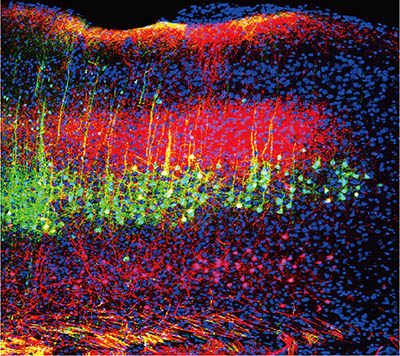
CREDIT: WENJIE ZHOU, U. OF SCIENCE AND TECHNOLOGY OF CHINA
NIDCR: Sound reduces pain in mice by lowering the activity of neurons in the brain’s auditory cortex (green and magenta) that project to the thalamus.
Music has seemingly magical qualities: Studies have shown it can relieve stress, improve memory recall, and even blunt pain. Precisely how music-induced analgesia works has remained a mystery, and researchers at NIDCR have uncovered new clues about how the brain turns sound into an effective painkiller. The findings were published in Science.
Previous functional magnetic-resonance imaging studies in humans have associated activity in certain brain areas with the pain-blunting effects of music. To find out exactly which pathways are involved, the NIDCR team turned to the mouse brain. They used noninfectious viruses coupled with fluorescent proteins to identify and trace neural connections. And to mimic music’s impact on the brain, scientists turned those brain circuits on and off while testing the pain threshold of mice that had inflamed paws.
The investigators at NIDCR and their collaborators at the University of Science and Technology of China (Hefei, China), pinpointed one route from the auditory cortex, the part of the brain that processes incoming sounds, to the somatosensory thalamus, which relays sensory information including pain. When exposed to low-intensity white noise or classical music, the cells at the thalamus end of that pathway fired less often and the mice felt less pain.
Researchers were then able to mimic music’s painkilling effects by turning off cells in this pathway without playing music. In contrast, activating the corticothalamic pathway blocked the sound-induced pain relief. With this discovery, scientists may be able to determine whether the animal findings apply to humans and develop new, safer alternatives to opioids for treating pain. (NIH authors: T. Li, L. Hayashi, and Y. Liu, Science 377:198–204, 2022; DOI:10.1126/science.abn4663)
[BY PETER MANZA, NIAAA]
NCI, NIAID: BACTERIAL POPULATIONS CONCENTRATE IN LUNG CANCER CELLS

CREDIT: CHEN ZHAO, NCI
A new spatial analysis from Chen Zhao and colleagues finds that the bacteria inside lung tumors concentrate in the cancer cells and are associated with activation of the beta-catenin signaling pathway. In this illustration, the lung tumors are depicted by the large green masses on the bottom right and top left; the bacteria and the associated beta-catenin signaling pathways are labeled by the red ovals.
Bacteria and other microbes within tumors are known to influence how lung cancer progresses and responds to treatment. But the exact location and nature of interaction between the microbiome and its host tumor cells is unknown. In a recent study, researchers at NCI, NIAID, and their colleagues used an advanced technology to map the local microbiome of lung-tumor tissue in 12 patients with early-stage lung cancer. The scientists discovered that lung-cancer cells harbor more bacteria than any other cell type, such as immune cells. Furthermore, tumor cells with high concentrations of bacteria also had increased expression of the beta-catenin gene, which is associated with a tumor-promoting signaling pathway.
The investigators used a new technique called spatial meta-transcriptomics that measured thousands of RNA molecules within the intact tumor samples. Those molecules signaled the presence of human genes, bacteria, and other microbes.
This study provides the first spatial microbiome map in the intratumor landscape. Lung cancer is the leading cause of cancer-related death in the United States and the new findings support the idea that therapies aimed at reducing bacterial load in the lungs might be beneficial for patients with lung cancer. (NIH authors: A. Wong-Rolle, J. L. Hor, A. Rajan, R. N. Germain, and C. Zhao, J Immunother Cancer 10:e004698, 2022; DOI:10.1136/jitc-2022-004698)
[BY AMRITA MANDAL, NICHD]
NIDA: NOVEL BRAIN MECHANISM INFLUENCES IMPULSIVE COCAINE-SEEKING IN RATS
Inhibiting certain acetylcholine receptors in the lateral habenula (LHb), an area of the brain that balances reward and aversion, made it harder for rats to resist seeking cocaine, according to a recent study led by NIDA researchers. The discovery may inform future treatments for cocaine-use disorder, for which there are currently no approved medications.
Using a rat model of impulsive behavior, rats were trained to self-administer cocaine: Pressing a lever led to injection of the drug. Then, the rodents were trained that cocaine was available only when lights were on, but not when the lights were off. Animals quickly learned to inhibit responses to obtain cocaine when it was not available.
The scientists then injected an experimental drug called AFDX-116, which blocks a specific type of acetylcholine receptor, known as M2Rs, into the LHb of rats. When M2Rs in the LHb were blocked, the trained rodents continued to seek cocaine even when the lights were off and drug was unavailable. Further experiments measured electrical activity changes in the LHb in response to other acetylcholine-like drugs, which identified cellular mechanisms by which AFDX-116 worked and confirmed the neural circuit’s role in enabling response inhibition for cocaine.
“While the immediate results of this study are related to cocaine seeking, there are also implications for impulsivity as it relates to other drugs as well as to psychiatric conditions like obsessive-compulsive disorder,” said lead author Carl Lupica. (NIH authors: C.I.C. Wolfe, E. Hwang, E.C. Ijomor, A. Zapata, A.F. Hoffman and C.R. Lupica, J Neurosci 42:5552–5563, 2022; DOI:10.1523/JNEUROSCI.0645-22.2022)
[BY SHIVALEE DUDUSKAR, NCI]
NEI: LOSS OF YOUTH PROTEIN MAY DRIVE AGING IN THE EYE
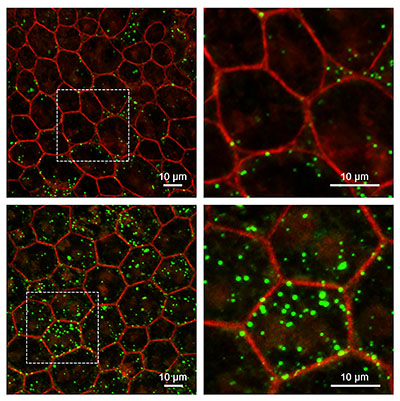
CREDIT: IVAN REBUSTINI, NEI
Retinal pigment epithelium (RPE) from mice without Serpin1 accumulate more lipids than wild-type mice. Super-resolution confocal microscopy of RPE tissue from wild-type (upper) and Serpin1-null (lower) mice. Detailed images on the right are magnified regions of the RPE tissue imaged on the left (dotted square area). RPE cell boundaries are stained in red, and accumulated lipids are stained in green.
Scientists have called pigment epithelium-derived factor (PEDF) the youth protein: It’s found in abundance in young retinas and declines during aging. In a recent study, NEI researchers and their colleagues discovered that a loss of PEDF led to a host of genetic alterations that may accelerate age-related changes in the retina, the light-sensitive tissue at the back of the eye. Diseases of the retina include age-related macular degeneration (AMD), which can cause blindness.
PEDF is expressed by a monolayer of cells behind the retina called the retinal pigment epithelium (RPE), which nourishes and supports photosensitive photoreceptor cells. When PEDF binds to its receptor, PEDF-R, this stimulates the breakdown of lipid molecules—a critical step toward supporting photoreceptor function. Accumulation of lipid deposits is a distinctive feature of AMD.
In this study, investigators used genetically modified mice that lacked the gene Serpin1, which encodes the PEDF protein. Those mice were found to have an increased expression of aging-related genes in their RPE cells and fewer PEDF receptors. Compared with healthy controls, the modified mice’s RPE cells also showed more accumulation of lipids. These findings highlight the protective role that PEDF plays in facilitating retinal homeostasis and preventing age-related changes. (NIH authors: I.T. Rebustini and S.P. Becerra, Int J Mol Sci 23:7745, 2022; DOI:10.3390/ijms23147745)
[BY DEVIKA BOSE, NEI]
NIEHS: SCIENTISTS SOLVE FIRST-EVER 3D STRUCTURE OF TWINKLE PROTEIN
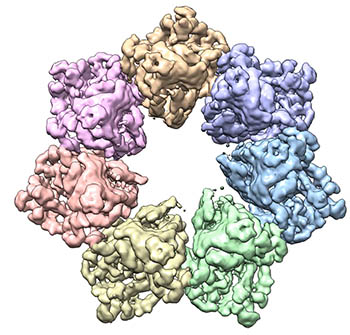
CREDIT: A.A. RICCIO, NIEHS
NIEHS: This rotating image shows the 3D structure that NIEHS researchers created of the twinkle protein. The researchers used Cryo-EM and other techniques to show how disease mutations on the protein can lead to mitochondrial diseases. The video zooms to the protein interface where many of the disease mutations occur. To see a video of the rotating image, go to https://www.youtube.com/watch?v=RrQtk_n_J28
NIEHS scientists have characterized the first-ever three-dimensional structure of the human twinkle protein, an enzyme that unwinds DNA and is implicated in inherited mitochondrial diseases. Mitochondrial diseases can lead to conditions including neurological disorders and liver failure and currently have few treatments. The new model allows researchers to successfully map disease-causing mutations in twinkle, which was impossible using previously available models.
Mitochondria are responsible for energy production and are vulnerable to mutations. Along with the nucleus, they are the only cellular organelles that have their own DNA. When mutations in the twinkle helicase enzyme prevent the accurate separation of the mitochondrial DNA double helix, multiple disease conditions can result.
To build their model, the researchers used a disease variant of twinkle called W315L and were able to observe the intricate inner structure of the protein with an advanced imaging technique known as cryoelectron microscopy. Computer simulations then helped the team understand why mutations affect the function of the helicase and result in disease.
“The arrangement of twinkle is a lot like a puzzle,” said lead author Amanda A. Riccio. “A clinical mutation can change the shape of the twinkle pieces, and they may no longer fit together properly to carry out the intended function.” The authors hope that the new information could lead to the development of treatments for mitochondrial diseases and allow clinicians to pinpoint the causes of mutations and help families make choices, including decisions about having more children. (NIH authors: A.A. Riccio, J. Bouvette, L. Perera, M.J. Longley, J.M. Krahn, J.G. Williams, R. Dutcher, M.J. Borgnia, and W.C. Copeland, PNAS 119:e2207459119, 2022; DOI:10.1073/pnas.2207459119)
[BY SATABDI NANDI, NIA]
NIAID: MONOCLONAL ANTIBODY PREVENTS MALARIA IN U.S. ADULTS
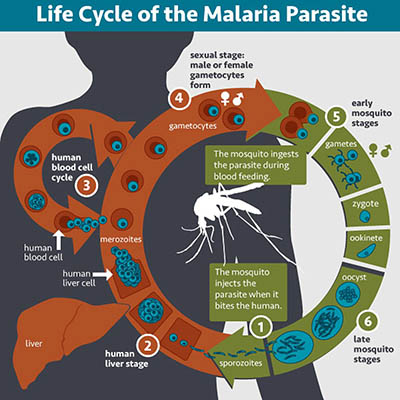
CREDIT: NIAID
This image shows the lifecycle of the malaria parasite in a person.
Prevention is the most effective strategy against malaria, the mosquito-borne disease caused by the Plasmodium parasite. Prophylaxis measures for the disease have long proved difficult, but scientists at NIAID’s Vaccine Research Center have developed a new monoclonal antibody, known as L9LS, that was found to be safe and efficacious in preventing malaria with just one subcutaneous injection. This study provides data showing that it may be feasible and cost effective to use a monoclonal antibody to prevent malaria in infants, young children, and pregnant women in regions where malaria is endemic.
L9LS works by interrupting the Plasmodium’s unique life cycle in humans. The antibody targets the parasite in the skin and blood before it has a chance to infect the liver, where it would normally reproduce prior to emerging in the blood to cause clinical symptoms.
In this phase I trial conducted at the NIH Clinical Center and Walter Reed Army Institute of Research (Silver Spring, Maryland), 18 participants received various does of L9LS. Two to six weeks after, participants were exposed to malaria in a carefully controlled setting known as controlled human malaria infection. L9LS protected against infection in 88% of participants, even in four of the five participants who received a single low subcutaneous dose. In contrast, all participants who did not receive L9LS developed malaria. Ongoing trials are underway in Mali and Kenya in infants and children to establish safety and efficacy of L9LS against seasonal and perennial infection. (NIH authors: R.L. Wu and R.A. Seder for the Vaccine Research Center 614 Study Team, N Engl J Med 387:397–407, 2022; DOI:10.1056/NEJMoa2203067)
[BY JONATHAN CHU, NIAID]
This page was last updated on Monday, September 19, 2022
