Research Briefs
Read about NIH scientific advances and discoveries by intramural scientists:
- NIA: ANTIBODY DRUGS MAY IMPROVE SYMPTOMS IN ALZHEIMER DISEASE
- NCI, NIEHS: UNLOCKING THE ORIGINS OF LUNG CANCER IN NEVER-SMOKERS
- NICHD: DRINKING JUICE BEFORE 6 MONTHS LINKED TO SUGARY BEVERAGE CONSUMPTION
- NINDS: INFECTION AFTER BRAIN INJURY IMPEDES BLOOD-VESSEL REPAIR
- NIAID: UNDERSTANDING HCV PROTEIN STRUCTURE MAY AID IN VACCINE DEVELOPMENT
- NIDA: DRAMATIC INCREASE IN METHAMPHETAMINE OVERDOSE RATES
- NCI: BLOOD TEST REVEALS WHEN BENIGN TUMORS TURN CANCEROUS IN COMMON GENETIC DISORDER
NIA: ANTIBODY DRUGS MAY IMPROVE SYMPTOMS IN ALZHEIMER DISEASE
CREDIT: STOCK (iStock image purchased by NIA)
NIA researchers recently found that monoclonal antibodies that target and bind to beta-amyloid may slightly improve cognitive function in people with Alzheimer disease. Shown: Digital illustration of cell antibodies.
A hallmark of Alzheimer disease (AD) is the buildup of a pathological protein known as beta-amyloid in the brain. The proteins clump together to form plaques that disrupt neuronal communication, ultimately resulting in cognitive decline. Scientists at NIA recently examined all phase 3 clinical trial data and found that monoclonal antibodies that target and bind to beta-amyloid may slightly improve cognitive function in people with AD.
Investigators conducted a meta-analysis encompassing results from 17 phase 3 clinical trials that assessed the effectiveness of five beta-amyloid antibodies: aducanumab, bapineuzumab, crenezumab, gantenerumab, and solanezumab. They found that aducanumab and solanezumab slightly improved participants’ thinking, memory, and performance of daily activities. Aducanumab also reduced the amount of beta-amyloid plaques as shown by positron-emission tomography imaging. The treatments were not without risk, however. Three of the drugs increased the chance of developing certain abnormalities such as fluid buildup or even bleeding in the brain.
The findings were published in Ageing Research Reviews and provide valuable data that will help researchers better assess the merits of beta-amyloid antibodies to treat AD. (NIH authors: K.I. Avgerinos, L. Ferrucci, and D. Kapogiannis, Ageing Res Rev 68:101339, 2021; DOI:10.1016/j.arr.2021.101339)
NCI, NIEHS: UNLOCKING THE ORIGINS OF LUNG CANCER IN NEVER-SMOKERS
CREDIT: NCI
Illustration of lungs made up of DNA sequences. A magnifying glass hovers over a portion of a DNA sequence showing a mutational change.
A large international study led by NCI researchers and their collaborators at NIEHS has found three distinct types of lung cancer in people who have never smoked.
Investigators characterized molecular changes in tumors from 232 people with no history of smoking who were diagnosed with non-small-cell lung cancer. Genomic analysis of the tumors revealed mutational signatures—patterns of mutations that give clues into what caused the cancer to develop—that were consistent with processes happening inside the body (such as faulty DNA repair or oxidative stress).
The analysis revealed three new subtypes of lung cancer in never-smokers, which the researchers assigned musical names correlating with the magnitude of genetic changes in the tumor. The most common “piano” subtype had the fewest mutations and grows slowly over many years. But it can have many different driver mutations and is difficult to treat. The “mezzo-forte” subtype grew faster and had specific chromosomal changes as well as mutations in a gene commonly altered in lung cancer. The “forte” subtype also grows quickly and exhibited whole-genome doubling, a genomic change that is often seen in lung cancers in smokers.
The findings may inform future treatments tailored to specific types of cancers. “We expect this detective-style investigation of genomic tumor characteristics to unlock new avenues of discovery for multiple cancer types,” said study co-author Stephen Chanock, director of NCI’s Division of Cancer Epidemiology and Genetics. (NIH authors: T. Zhang, W. Zhao, P.H. Hoang, R. Lokanga, M. Kebede, M. Li, D. Hirsch, K. Heselmeyer-Haddad, A. Hutchinson, A. Hutchinson, M. Olanich, S.M. Lawrence, P. Lenz, P.M.S. Bhawsar, J. Sang, J. Kim, L. Mendoza, N. Saini, L.J. Klimczak, N. Cole, D.R. Stewart, J. Choi, K.M. Brown, N.E. Caporaso, S.H. Wilson, Y. Pommier, Q. Lan, N. Rothman, J.S. Almeida, T. Ried, M. Garcia-Closas, J. Shi, B. Shu, D.A. Gordenin, S.J. Chanock, and M.T. Landi, Nat Genet 53:1348–1359, 2021; DOI:10.1038/s41588-021-00920-0)
NICHD: DRINKING JUICE BEFORE 6 MONTHS LINKED TO SUGARY BEVERAGE CONSUMPTION

CREDIT: STOCK IMAGE
In 2017, the American Academy of Pediatrics recommended that children younger than one year old should not have 100% fruit juice introduced into their diet. Early juice consumption is suspected to be associated with obesity and tooth cavities in later childhood, and a recent NICHD-led study has shed new light on this theory.
Researchers analyzed data from an existing prospective birth cohort study of 4,067 children and their parents. The study asked parents to answer periodic questionnaires about when they first introduced juice to their child and collected sociodemographic information. Parents then reported their child’s juice, soda, water, and milk intakes at 24 months, 30 months, 36 months, and seven years.
Across all age intervals, the investigators found that children introduced to juice before they were 6 months old drank more juice and soda and less water than children given juice after they were one year old. Markers of socioeconomic risk such as younger parenthood, lower educational attainment, and smoking during pregnancy were related to earlier juice introduction.
The authors concluded that early introduction of juice in infancy may predispose children to a preference for sweet tastes. Furthermore, the findings suggest behaviors that exacerbate health disparities begin early in life, and more research is needed to identify drivers of these associations. (NIH authors: S.L. Robinson, R. Sundaram, D.L. Putnick, J.L. Gleason, and E.H. Yeung, J Nutr 2021; DOI:10.1093/jn/nxab260)
NINDS: INFECTION AFTER BRAIN INJURY IMPEDES BLOOD-VESSEL REPAIR
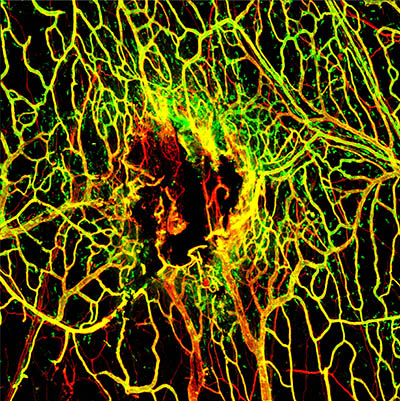
CREDIT: D.B. MCGAVERN LAB, NINDS
Viral Infection Slows Blood Vessel Repair After TBI: Seven days after mTBI, the blood vessels (stained in red) in the tissues around the brain are not completely repaired. A marker for intact vessels was used (labeled in green) to distinguish fully functional blood vessels (yellow) from ones that are still damaged (red).
In a study published in Nature Immunology, NINDS researchers found that infections hindered blood-vessel repair after injury to brain tissue as well as the meninges, the protective sheath that covers the brain. Injuries to the blood vessels in the brain and meninges, such as traumatic brain injury (TBI) and stroke, are a major cause of disability and death worldwide. Systemic infections (viral, bacterial, or fungal) are common in hospitalized patients with brain injuries, and the NINDS team found that these infections can distract the immune system, delaying essential repair of blood vessels and leading to worse outcomes.
Using a mouse model previously developed for mild TBI, investigators led by Dorian McGavern found that some immune cells stopped repairing the injury site after infection, resulting in far slower healing in infected mice. This effect was temporary, as infected mice eventually did heal unless a second infection was introduced. Additional experiments revealed that a class of proteins called type I interferons (IFN-I) disrupt the repair process after infection by shifting the focus of the immune response—a new mechanistic insight that may help guide future therapeutic interventions.
Researchers then tested how infection influences recovery from cerebrovascular injury (CVI) using a second mouse model. They found a similar delay in blood-vessel repair, with IFN-I activity again playing a large role. And in the case of the infected CVI mice, the slower recovery led to permanent brain damage. “These findings highlight the utmost importance [of] quickly identifying and treating infections in patients,” said McGavern. (NIH authors: P. Mastorakos, M.V. Russo, T. Zhou, K. Johnson, and D.B. McGavern, Nat Immunol 22:1280–1293, 2021; DOI:10.1038/s41590-021-01012-1)
NIAID: UNDERSTANDING HCV PROTEIN STRUCTURE MAY AID IN VACCINE DEVELOPMENT
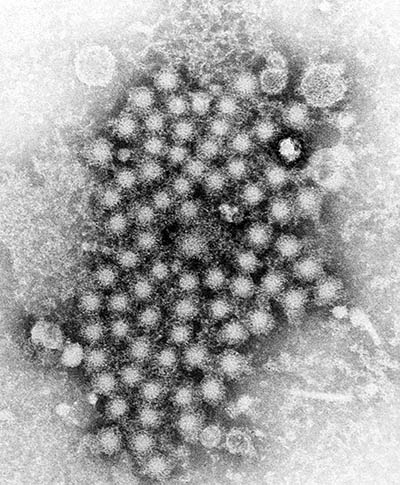
CREDIT: CDC/E.H. COOK, JR.
A transmission electron microscopic image of hepatitis virus particles
Hepatitis C virus (HCV) is one of the most common bloodborne infections in the United States, affecting an estimated 2.4 million people. Chronic infections can lead to liver disease, cancer, cirrhosis, and death. HCV is usually transmitted through blood, such as during childbirth or when sharing drug-injection equipment.
In a study published in Nature, a NIAID-led team of researchers described the mechanics behind HCV infection of human cells. Their findings build on prior research suggesting that a protein on the surface of HCV known as E2 engages with a receptor on liver cells called CD81, allowing the virus to enter its host.
The investigators studied how the two proteins interact under different conditions. They found that an acidic environment promoted HCV E2 binding to the CD81 receptor. And once associated, the viral protein changed shape, facilitating infection by bringing the virus closer to the host cell membrane.
This discovery may lead to a future vaccine against HCV, said the authors. A potential vaccine could trigger the host to produce specific antibodies that block HCV E2 binding with CD81, preventing HCV infection. (NIH authors: A. Kumar, R.A. Hossain, W. Bu, Y. Wang, A.D. Dearborn, J.I. Cohen, and J. Marcotrigiano, Nature 598:521–525, 2021; DOI:10.1038/s41586-021-03913-5)
NIDA: DRAMATIC INCREASE IN METHAMPHETAMINE OVERDOSE RATES
Methamphetamine overdose deaths have escalated nationwide in recent years. To better understand this concerning trend, NIDA researchers completed a cross-sectional analysis of a nationally representative survey and national overdose mortality data. They assessed patterns of methamphetamine use and methamphetamine-involved overdose deaths in individuals aged 18—64. The study showed that use of the drug increased 43% between 2015 and 2019, but overdose deaths rose from 5,526 to 15,489, an 180% increase. In parallel with rising overdose mortality, survey respondents reported riskier patterns of use such as injected methamphetamine, co-use with cocaine, and/or increased rates of methamphetamine use disorder (MUD), a psychiatric condition characterized by compulsive drug taking despite negative consequences.
Moreover, the study found that populations using methamphetamine diversified in the time period analyzed. While middle-aged white individuals have historically been at greatest risk for methamphetamine use, populations with socioeconomic risk factors and comorbidities (such as HIV, hepatitis B or C, and depression) are increasingly being affected. The highest prevalence of methamphetamine use and MUD was reported in American Indians and Alaska Natives. Further analysis showed a 10-fold increase in the prevalence of MUD among Black people who did not inject the drug and a 4-fold increase in young adults aged 18—23, a time period that is critical for brain development.
“What makes these data even more devastating is that currently, there are no approved medications to treat methamphetamine use disorder,” said study co-author Emily Einstein. “NIDA is working to develop new treatment approaches, including safe and effective medications urgently needed to slow the increase in methamphetamine use, overdoses, and related deaths.” (NIH authors: B. Han, W.M. Compton, E.B. Einstein, and N.D. Volkow, JAMA Psychiatry 2021; DOI:10.1001/jamapsychiatry.2021.2588)
NCI: BLOOD TEST REVEALS WHEN BENIGN TUMORS TURN CANCEROUS IN COMMON GENETIC DISORDER
NCI scientists and collaborators at Washington University (St. Louis) have developed a blood test that allows for early cancer detection in people with neurofibromatosis type 1 (NF1). NF1 is a genetic disorder that causes the development of benign tumors along nerves, which can turn into an aggressive cancer called malignant peripheral nerve sheath tumor (MPNST). Current standards (such as a biopsy or positron-emission tomography scan) to differentiate MPNST from benign tumors can be challenging and impractical, and the researchers conducted a study to determine whether blood markers could have diagnostic potential.
The study sampled blood from 53 people: 23 with benign tumors, 14 with MPNST, and 16 healthy volunteers. Cell-free DNA (DNA shed from cells into the blood) was then analyzed with whole-genome sequencing to search for differences in genetic material among the groups.
Samples from MPNST donors had several distinguishing markers including shorter fragments of cell-free DNA. The research team also discovered that the proportion of cell-free DNA originating from the tumor, known as tumor fraction, was greater in MPNST patients compared with the other groups. Comparing these differences allowed investigators to distinguish between MPNST and benign tumors with 86% accuracy. Additionally, tumor fraction was found to correlate with tumor size and could be used to monitor treatment effectiveness.
The team is now working to increase the accuracy of the current test and planning to conduct the study with more patients. They hope the technology can be further developed to improve early detection and monitoring of other cancer-predisposing genetic disorders. (NIH authors: R.T. Sundby, H. Lei, L. Hoffman, M. Spencer, B.C. Widemann, and J.F. Shern, PLoS Med 18:e1003734, 2021; DOI:10.1371/journal.pmed.1003734)
This page was last updated on Monday, January 31, 2022
